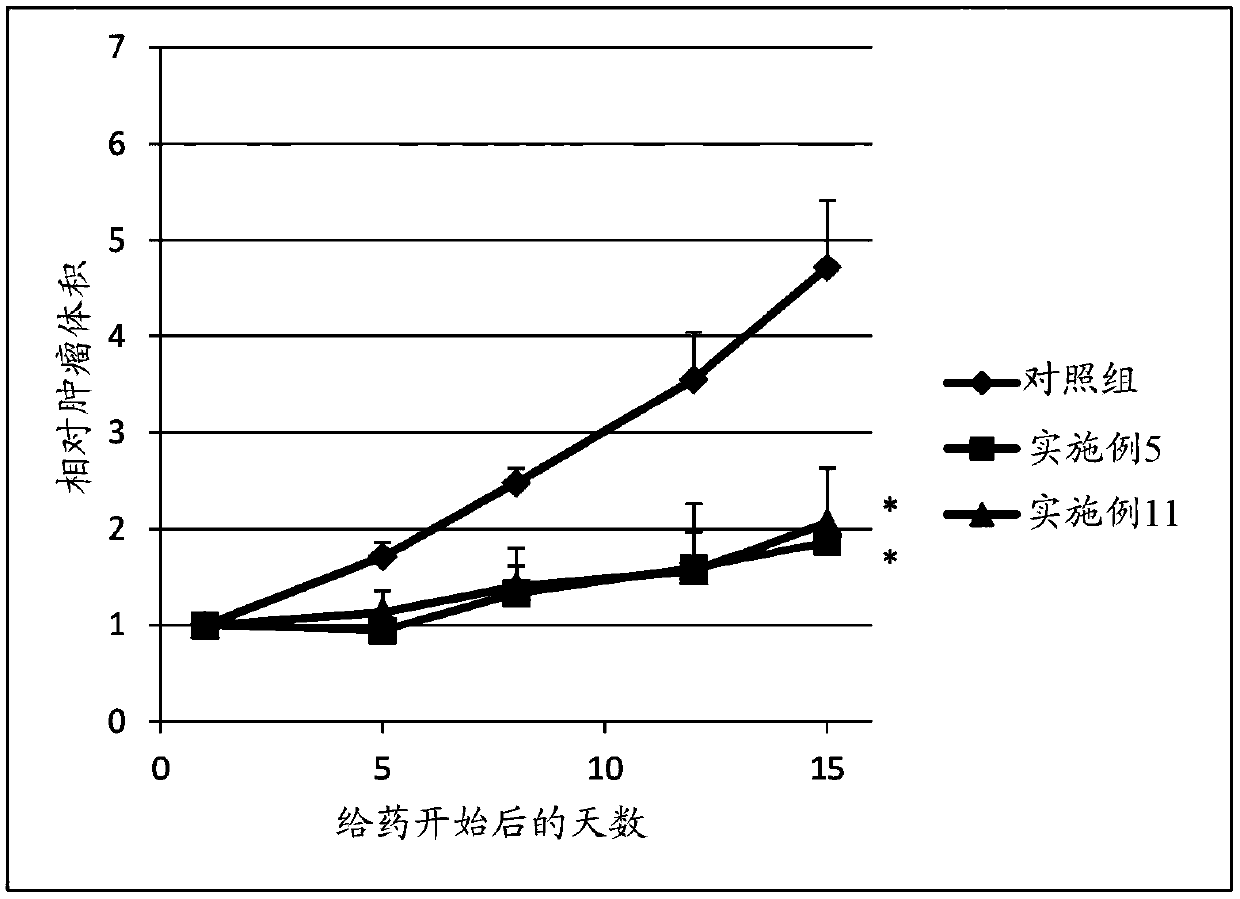
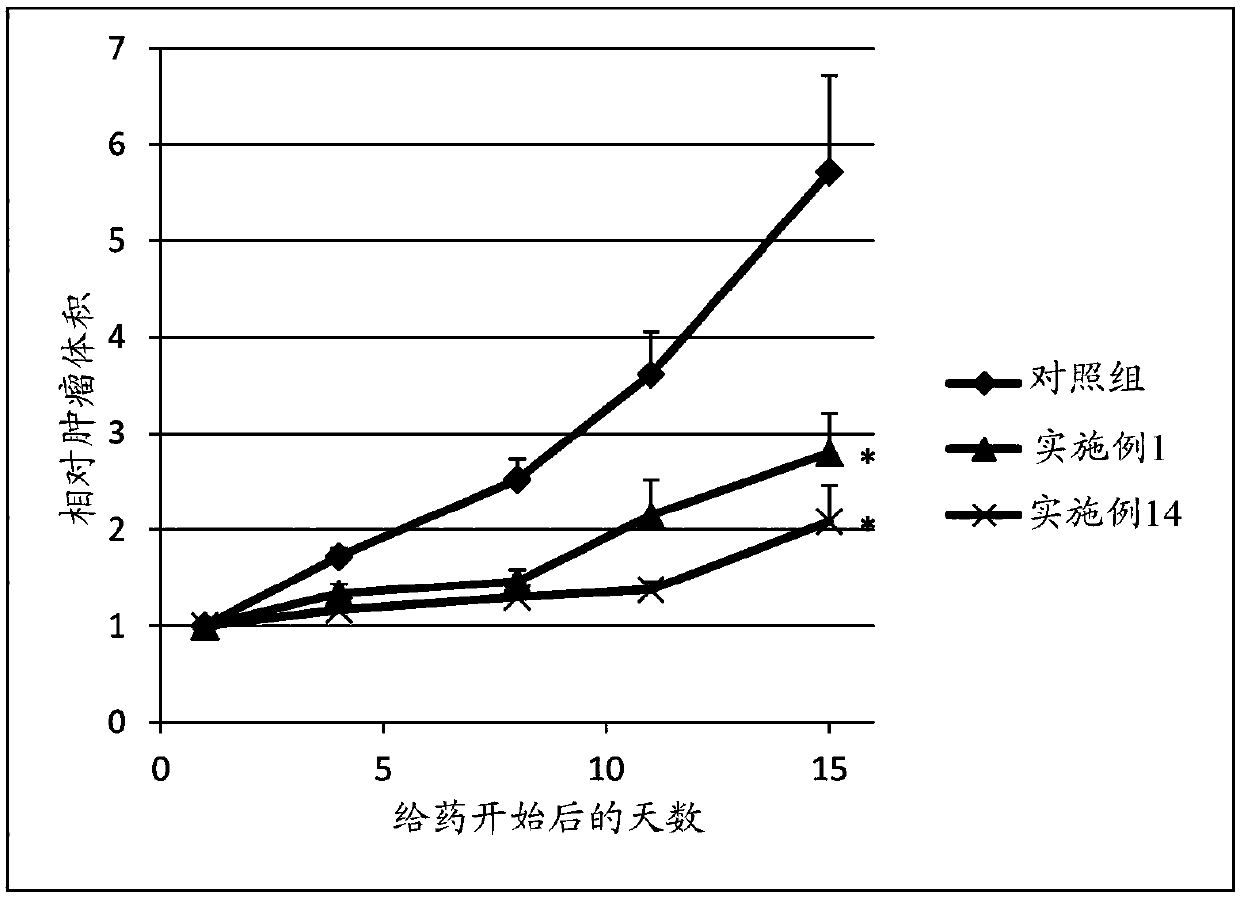
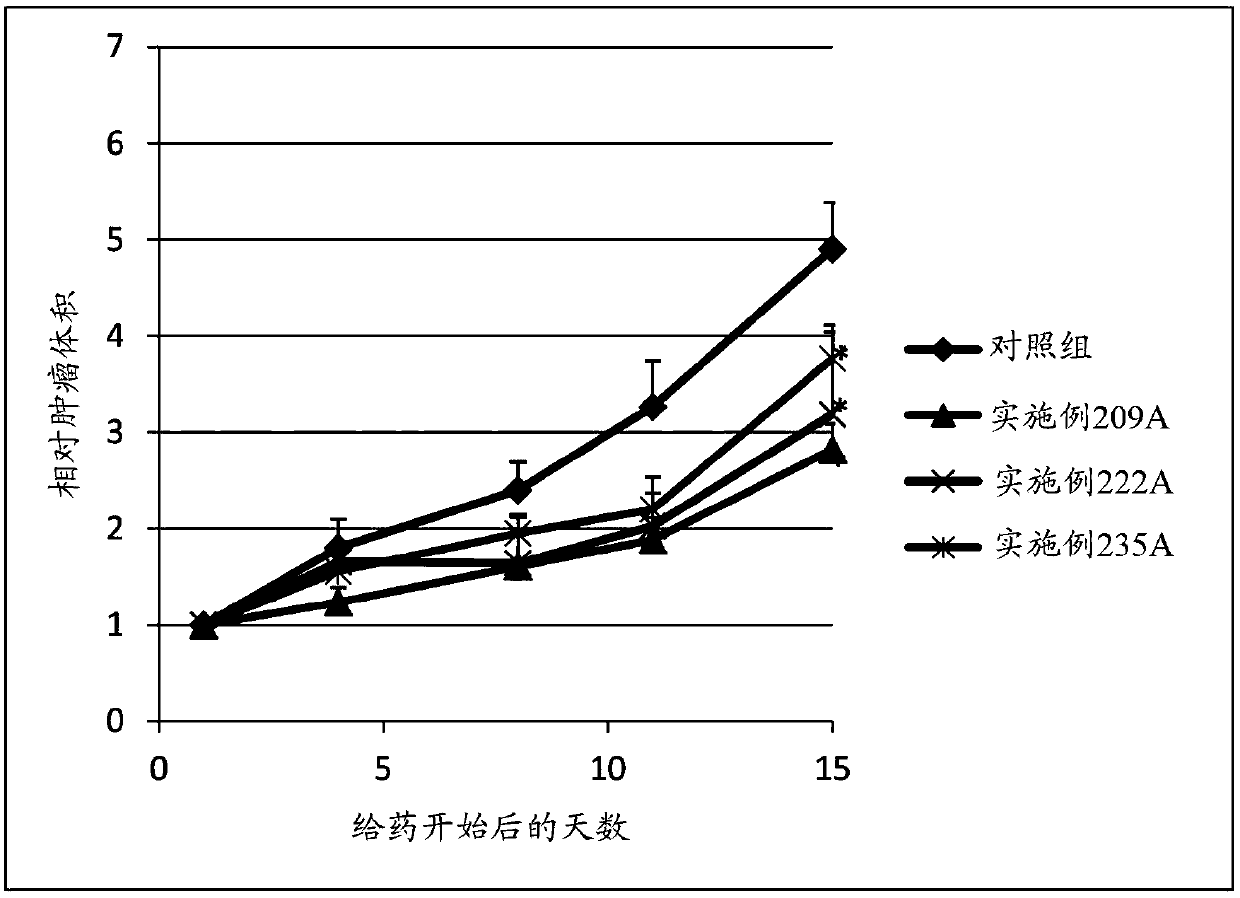
Sulfonamide compound or salt thereof
A technology of compounds and oxygen atoms, applied in the fields of organic chemistry, drug combination, organic active ingredients, etc., can solve the problems of no indication of RNR inhibitory activity and anti-tumor effect, no physiological activity of compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0561] The present invention will be described in more detail below through examples and test examples, but the present invention is not limited to these examples.
[0562] Regarding various reagents used in Examples, commercially available products were used unless otherwise specified. For silica gel column chromatography, use the SNAP-ULTRA (registered trademark) silica gel prepacked column produced by Baitaiqi Company, or use the SNAP KP-C18-HS (registered trademark) silica gel prepacked column produced by Baitaiqi Company for reverse-phase silica gel column chromatography. Packed column. The reverse-phase separation HPLC column chromatography was implemented according to the following conditions. Appropriately set the injection volume and gradient to implement.
[0563] Chromatographic column: YMC-Actus Triart C18, 30×50mm, 5μm
[0564] UV detection: 254nm
[0565] Column flow rate: 40mL / min
[0566] Mobile phase: water / acetonitrile (0.1% formic acid)
[0567] Inject...
reference example A1
[0605] Reference Example A1 2-(1-bromoethyl)-1-fluoro-3,4-dimethylbenzene
[0606] [chemical 12]
[0607]
[0608] (Step 1) 1-(6-fluoro-2,3-dimethylphenyl)ethanol
[0609] Add methylmagnesium bromide in diethyl ether (3.0M, 70mL) dropwise to a THF solution (300mL) of 6-fluoro-2,3-dimethylbenzaldehyde (22.0g) at 0°C, and then The reaction solution was stirred for 1 hour. Under ice cooling, saturated ammonium chloride aqueous solution (150 mL) was added dropwise, and ethyl acetate (200 mL) was added to separate the layers. The organic layer was washed successively with hydrochloric acid (1M, 200mL), water (200mL) and saturated brine (200mL), then dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 1-(6-fluoro-2,3-di Methylphenyl)ethanol (23.7 g).
[0610] (step 2)
[0611] Phosphorus tribromide (26.5 mL) was added dropwise to a chloroform solution (120 mL) of 1-(6-fluoro-2,3-dimethylphenyl) ethanol (23.7 g) obtained in the above step...
reference example A2~A41
[0613] In the same manner as in steps 1 and 2 of Reference Example A1, the starting material aldehyde was reacted with methylmagnesium bromide and then reacted with phosphorus tribromide to obtain the compounds of Reference Examples A2 to A41 shown below. Among them, the compounds of Reference Examples A40 and A41 were produced in the same procedure using ethylmagnesium bromide and methyl-d3-magnesium iodide, respectively, instead of methylmagnesium bromide.
[0614] [Table 1-1]
[0615]
[0616]
[0617] [Table 1-2]
[0618]
[0619]
[0620] [Table 1-3]
[0621]
[0622]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com