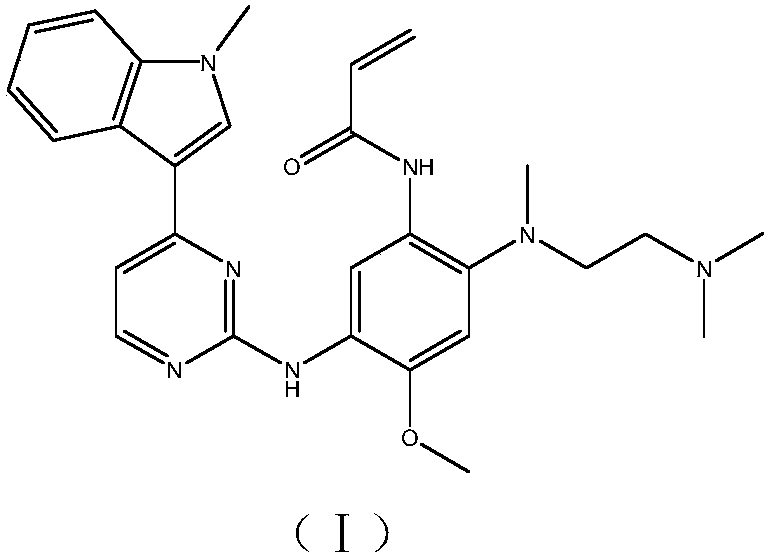
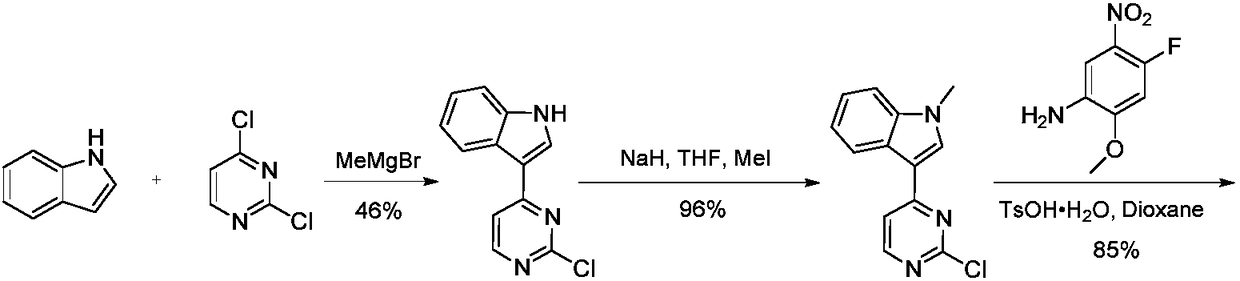
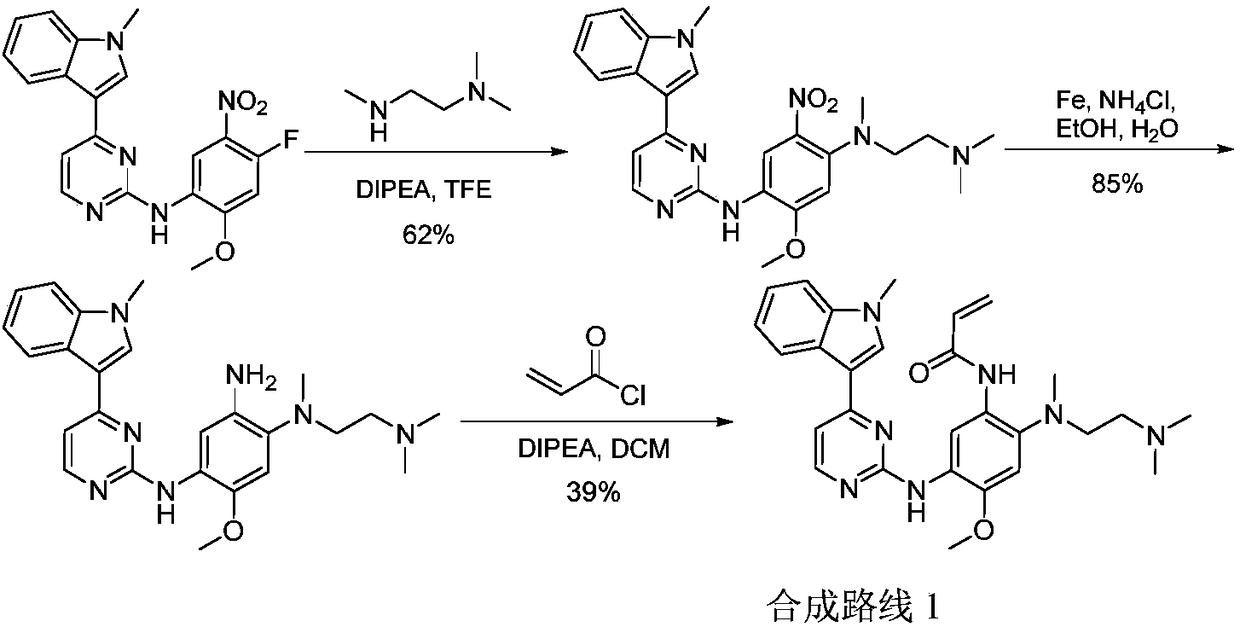
Preparation method of osimertinib intermediate
A technology for intermediates and compounds, applied in the direction of organic chemistry, etc., can solve the problems of low total yield and high cost of raw materials, and achieve the effects of low cost, high reaction selectivity and environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Preparation of 1-(2-nitro)phenyl-4,4-dimethoxy-2-butanone (IV)
[0064] Into a 500 ml four-neck flask, add 68.5 g (0.5 mole) o-nitrotoluene, 69.0 g (0.6 mole) 3,3-dimethoxypropionitrile, 0.8 g piperidine, react at 110 ° C for 4 hours, gas phase detection The reaction is complete. Reduce to 25°C, add 50g of 10% ammonium chloride aqueous solution, 250g of dichloromethane, stir at 25-30°C for 2 hours. The reaction solution was transferred to a separatory funnel, and the layers were separated. The aqueous layer was extracted twice with 50 g of dichloromethane each time, and the organic phases were combined. The organic phase was washed 3 times with water, each time with 20 ml of water, dried with 10 g of anhydrous sodium sulfate for 4 hours, filtered, and after the filtrate recovered dichloromethane, it was distilled under reduced pressure (80-95°C / 5mmHg) to obtain a light yellow viscous liquid 1 -(2-nitro)phenyl-4,4-dimethoxy-2-butanone (IV) 121.3 g, gas phase...
Embodiment 2
[0068] Example 2: Preparation of 1-(2-nitro)phenyl-4,4-dimethoxy-2-butanone (IV)
[0069] In a 500 ml four-neck flask, add 68.5 grams (0.5 moles) of o-nitrotoluene, 69.0 grams (0.6 moles) of 3,3-dimethoxypropionitrile, 1.0 grams of DBU, react at 100 ° C for 4 hours, and detect the reaction by gas phase complete. Reduce to 25°C, add 50g of 10% ammonium chloride aqueous solution, 250g of dichloromethane, stir at 25-30°C for 2 hours. The reaction solution was transferred to a separatory funnel, and the layers were separated. The aqueous layer was extracted twice with 50 g of dichloromethane each time, and the organic phases were combined. The organic phase was washed 3 times with water, each time with 20 ml of water, dried with 10 g of anhydrous sodium sulfate for 4 hours, filtered, and after the filtrate recovered dichloromethane, it was distilled under reduced pressure (80-95°C / 5mmHg) to obtain a light yellow viscous liquid 1 -(2-nitro)phenyl-4,4-dimethoxy-2-butanone (IV) 120...
Embodiment 3
[0070] Example 3: Preparation of 1-(2-nitro)phenyl-4,4-diethoxy-2-butanone (IV)
[0071] In a 500 ml four-neck flask, add 68.5 g (0.5 mole) o-nitrotoluene, 85.8 g (0.6 mole) 3,3-diethoxypropionitrile, 1.0 g morpholine, react at 110°C for 3 hours, and gas phase detection The reaction is complete. Reduce to 25°C, add 50g of 10% ammonium chloride aqueous solution, 250g of dichloromethane, stir at 25-30°C for 2 hours. The reaction solution was transferred to a separatory funnel, and the layers were separated. The aqueous layer was extracted twice with 50 g of dichloromethane each time, and the organic phases were combined. The organic phase was washed 3 times with water, each time with 20 ml of water, dried with 10 g of anhydrous sodium sulfate for 4 hours, filtered, and after the filtrate recovered dichloromethane, it was distilled under reduced pressure (90-105°C / 5mmHg) to obtain a light yellow viscous liquid 1 -(2-nitro)phenyl-4,4-diethoxy-2-butanone (IV) 131.5 g, gas phase p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com