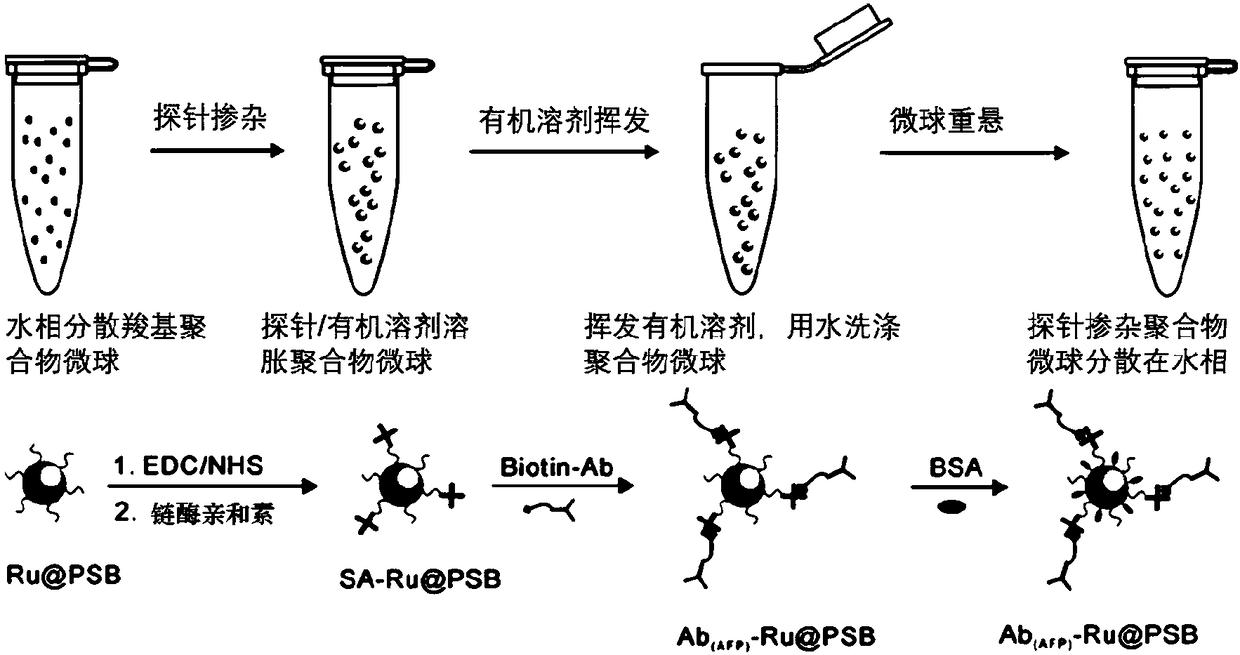
Potential-resolved electrochemiluminescence-based antigen detection method
A technology of antigen detection and electrochemistry, which is applied in the direction of chemiluminescence/bioluminescence, and analysis through chemical reaction of materials, can solve the problems of complex modulation, achieve high sensitivity, improve consumption, and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
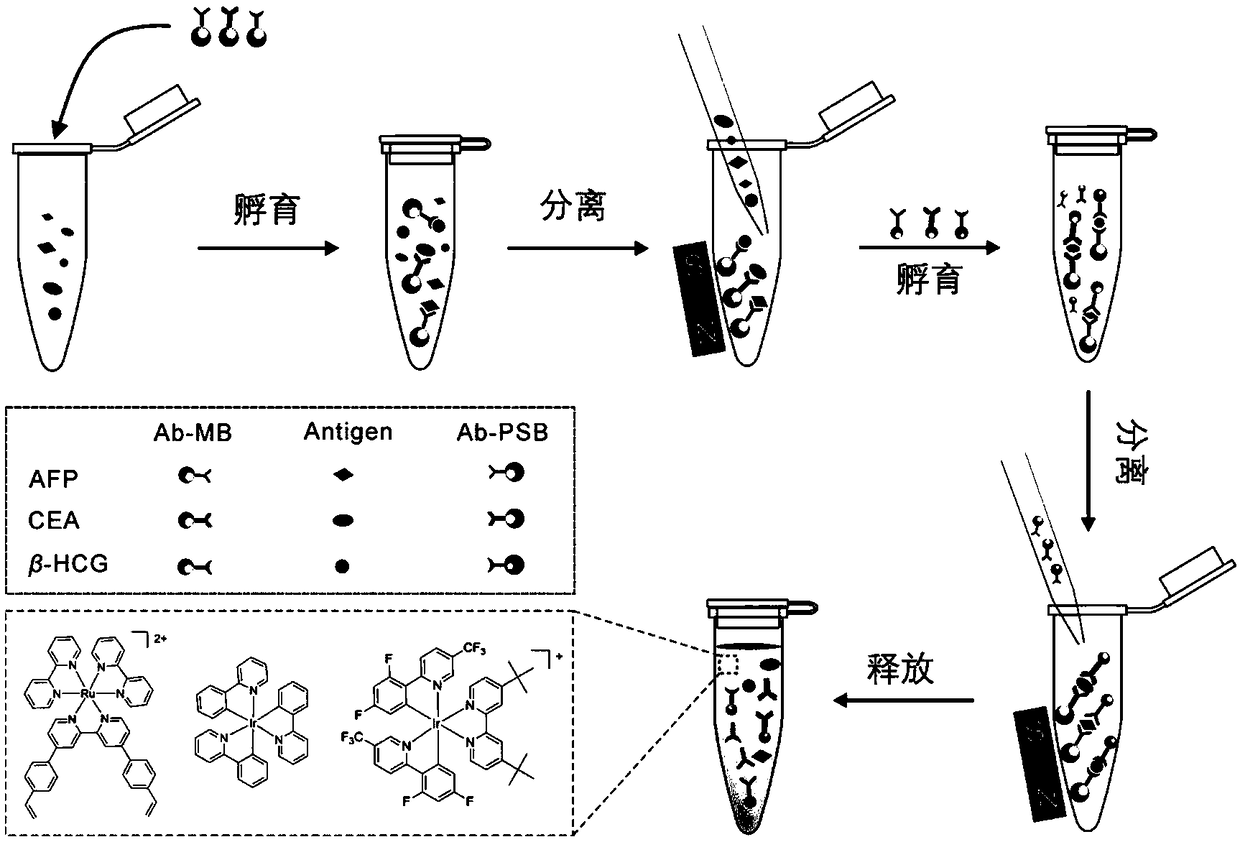
[0068] Such as image 3 As shown, alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) and human chorionic gonadotropin (β-HCG) were detected simultaneously.
[0069] The simulated sample for simultaneous detection of multiple indicators is the PBS solution containing CEA, AFP and β-HCG. According to the clinical detection values of tumor markers, the final concentrations of CEA, AFP and β-HCG in the detection system were adjusted to 5 ng / mL, 25 ng / mL and 5 mIU / mL, respectively. The process of simultaneous detection of multiple tumor markers mainly includes steps such as incubation, magnetic separation, washing, and detection. The specific operation is as follows:
[0070] (1) Add 100 μL of magnetic bead solutions labeled with three kinds of capture antibodies (the concentration of magnetic beads is 1 mg / mL) into 300 μL of PBS mock sample, and incubate for 30 min in a constant temperature shaker at 37°C. After incubation, place the centrifuge tube on the magnetic separ...
Embodiment 2
[0077] Add any pair of detection antibody-labeled polymer microspheres and capture antibody-labeled magnetic beads into the simulated sample to investigate the selective detection performance of a single indicator in multi-index samples.
[0078] Taking the selective detection of AFP as an example, take 100μL containing 1mg / mL Ab (AFP) -MB PBS buffer solution and 200 μL 0.5% BSA / PBS solution were added to 300 μL of the above sample, and incubated at 37° C. for 30 min. Separate / wash, repeat 3 times; then add 300 μL 0.5% BSA / PBS solution, 25 μL 1mg / mL Ab (AFP) -Ru@PSB and 50 μL of 0.5% BSA / PBS solution, mix well, and incubate at 37° C. for 30 minutes. Separation / washing was repeated 5 times. After vacuum drying, 100 μL of acetonitrile solution was added and ultrasonically swelled. When detecting CEA and β-HCG, the concentration of probe molecules added was 10 mg / mL, and other operation steps were consistent with the above-mentioned procedures.
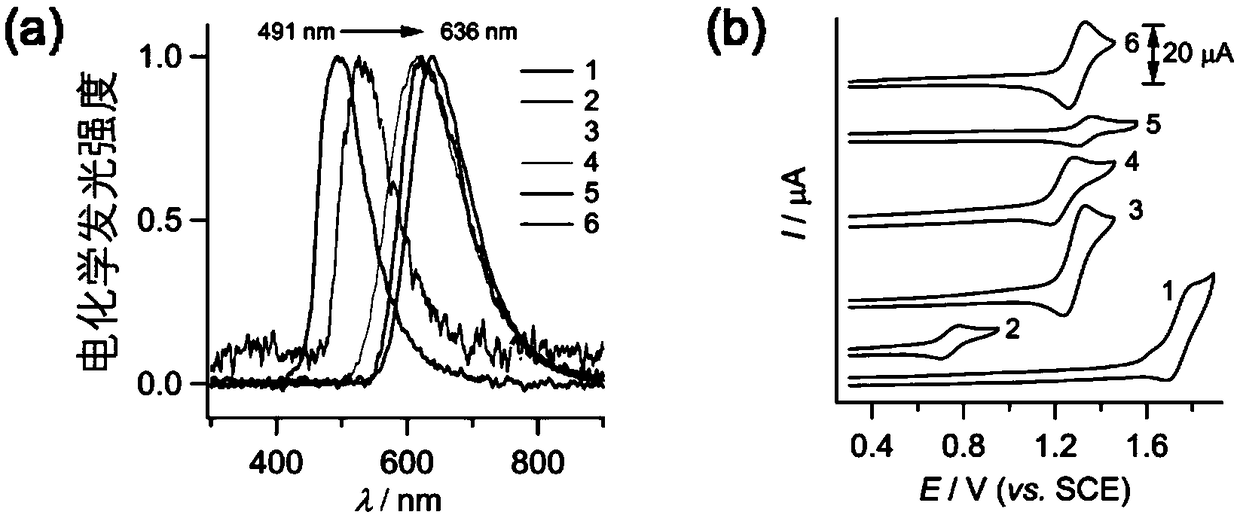
[0079] Figure 6 Shown are th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com