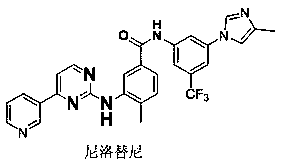
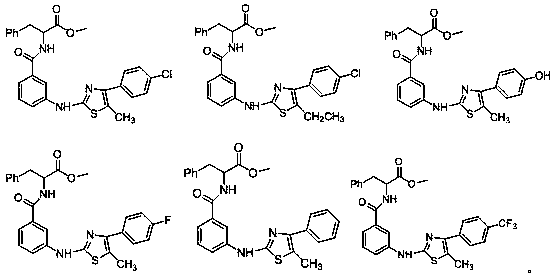
Thiazol-aminobenzamide acetate derivative and application thereof
A technology of alkyl and methyl, applied in the field of medicinal chemistry, can solve problems such as drug resistance that cannot be effectively solved, and achieve the effect of broadening the scope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 (3-((5-methyl-4-phenylthiazol-2-yl)amino)benzoyl)glycine methyl ester
[0059] Step a:
[0060]
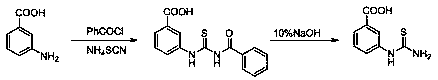
[0061] Add 11.4341 g (0.12 mol) of ammonium thiocyanate and 20 mL of acetone into a 100 mL oblique-necked reaction flask equipped with mechanical stirring and a condenser, and stir evenly through mechanical stirring. 16.8034 g (0.13 mol) of benzoyl chloride was added dropwise (dropped in 10 min), and the solution changed from clear to white turbid. Heat to reflux, add 14.1147 g (0.10 mol) m-aminobenzoic acid in 4 batches, monitor the reaction process by TLC (ethyl acetate:petroleum ether = 4:1), and complete the reaction in 8 h. Cool, filter, and dry the obtained solid to obtain 28.0041 g of light yellow powder 3-(3-benzoylthioureido)benzoic acid, m.p. 184 ~ 186 ℃.
[0062] Add 0.9913 g (0.12 mol) of 3-(3-benzoylthioureido)benzoic acid and 33 mL of 10% NaOH into a 100 mL oblique-necked reaction flask with a condenser, the measured pH=13, magnetic stirring, an...
Embodiment 2
[0069] Example 2 (3-((5-methyl-4-(p-tolyl)thiazol-2-yl)amino)benzoyl)glycine methyl ester
[0070]
[0071] The operation was the same as above to obtain 0.3018g of white powder, yield 68.23%, m.p.187 ~ 188°C. 1 H NMR (DMSO-D 6 , 400 MHz), δ : 2.36 (s, 3H, CH 3 ), 2.42 (s, 3H, CH 3 ), 3.67(s, 3H, OCH 3 ), 4.02 (d, J = 4.0 Hz, 2H, NHCH 2 ), 7.26-8.07 (m, 8H, 2×C 6 h 4 ), 8.90 (t, J = 4.0, 1H, CONH), 10.30 (s, 1H, NH).
Embodiment 3
[0072] Example 3 3-((4-(4-chlorophenyl)-5-methylthiazol-2-yl)amino)benzoyl)glycine methyl ester
[0073]
[0074] The operation was the same as above to obtain 0.3016g of yellow powder, yield 70.44%, m.p.158 ~ 159°C. 1 H NMR (CDCl 3 , 400MHz), δ : 1.41 (s, 3H, CH 3 ), 2.44 (s, 3H, OCH 3 ), 4.39(d, J = 4.0 Hz, 2H, NHCH 2 ), 7.34-8.01 (m, 8H, 2×C 6 h 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com