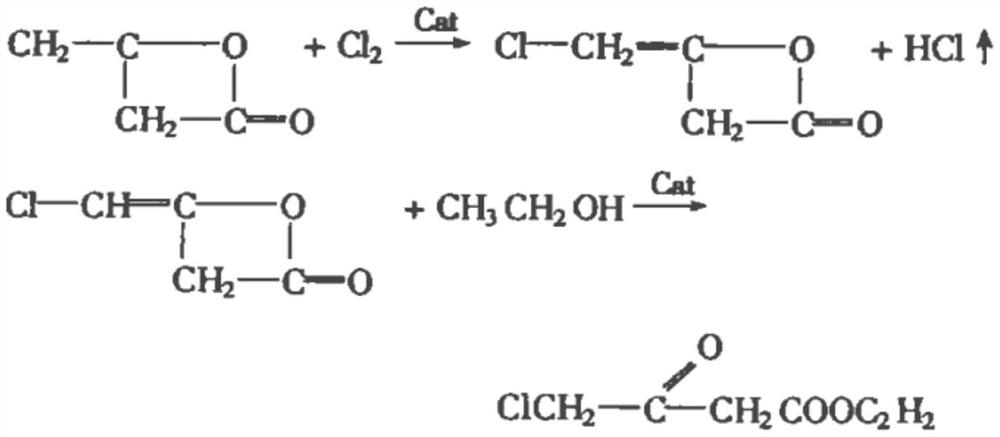
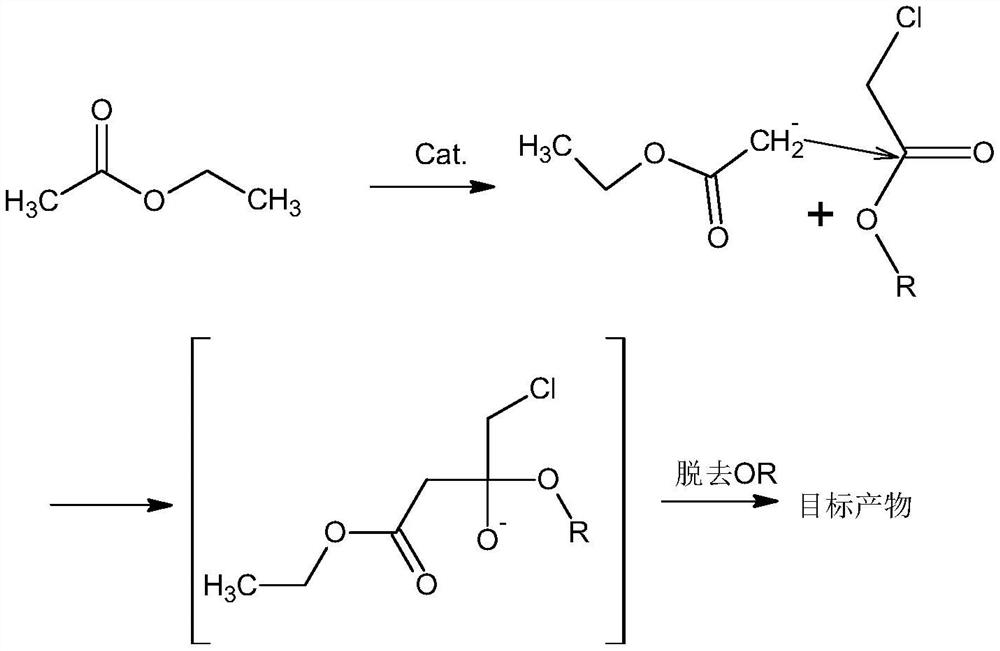
The synthetic method of oxiracetam intermediate 4-chloroacetoacetate ethyl
A technology of ethyl chloroacetoacetate and ethyl acetate, which is applied in the field of synthesis of oxiracetam intermediate 4-ethyl chloroacetoacetate, can solve the problems of low product yield, target product decomposition loss, separation and purification difficulties, etc. , to achieve high product purity, increase the probability of "collision" and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0031] 1) Mix the ethyl acetate, the catalyst and DMSO evenly, pass in argon, control the pressure to 4.8 atmospheres, control the temperature to 128°C, add the solution of ethyl chloroacetate and ethanol dropwise, and control the addition time to 21min; After finishing, raise the reaction temperature to 152° C., raise the pressure to 9 atmospheres, and then continue to react for 22 hours to complete the reaction;
[0032] The preparation method of the catalyst used is as follows: the molecular sieve of type 5A is soaked in 20% potassium carbonate aqueous solution for 50 hours, dried in the air, and then activated at 450 DEG C to obtain.
[0033] The consumption ratio of ethyl acetate and catalyzer is 1g:0.31g, the consumption ratio of ethyl acetate and DMSO is 1g:6.8ml, the consumption ratio of ethyl acetate and ethyl chloroacetate is 1mol:0.97mol, ethyl chloroacetate and The usage ra...
Embodiment 2
[0036] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0037] 1) Mix ethyl acetate, catalyst and DMF, feed nitrogen, control the pressure to 3 atmospheres, control the temperature to 110°C, add the solution of methyl chloroacetate and methanol dropwise, and control the addition time to 15min; , raising the reaction temperature to 145°C, raising the pressure to 8 atmospheres, and then continuing to react for 15 hours to complete the reaction;
[0038] The preparation method of the catalyst is as follows: immerse the 4A type molecular sieve in 15% potassium carbonate or sodium carbonate aqueous solution for 2 days, then dry it, and then activate it at 350°C.
[0039] The consumption ratio of ethyl acetate and catalyzer is 1g:0.25g, and the consumption ratio of described ethyl acetate and DMF is 1g:5.6ml, and the consumption ratio of described ethyl acetate and methyl chloroacetate is 1mol:0.95mol, so The consumption ratio of methyl chloroace...
Embodiment 3
[0042] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0043] 1) Mix the ethyl acetate, the catalyst and DMSO evenly, pass in argon, control the pressure to 5 atmospheres, control the temperature to 135°C, add the solution of ethyl chloroacetate and ethanol dropwise, and control the addition time to 25min; After finishing, raise the reaction temperature to 160° C., raise the pressure to 10 atmospheres, and then continue to react for 25 hours to complete the reaction;
[0044] The preparation method of the catalyst is as follows: immerse the 5A type molecular sieve in 25% potassium carbonate or sodium carbonate aqueous solution for 3 days, then dry it, and then activate it at 470 DEG C to obtain it.
[0045] The consumption ratio of ethyl acetate and catalyzer is 1g:0.33g, and the consumption ratio of described ethyl acetate and DMSO is 1g:7.5ml, and the consumption ratio of described ethyl acetate and ethyl chloroacetate is 1mol:1mol, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com