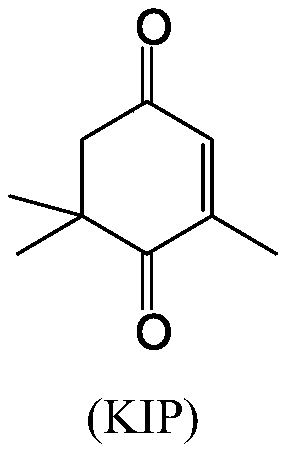
A kind of synthetic method of oxyisophorone
A technology of oxyisophorone and synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of dangerous operating conditions and high cost of reaction process, and achieve less discharge of three wastes, Process-friendly, high-yield results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
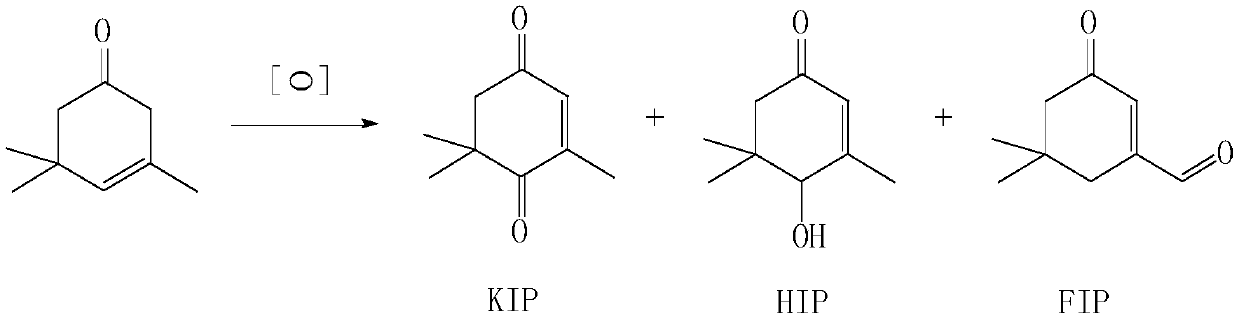
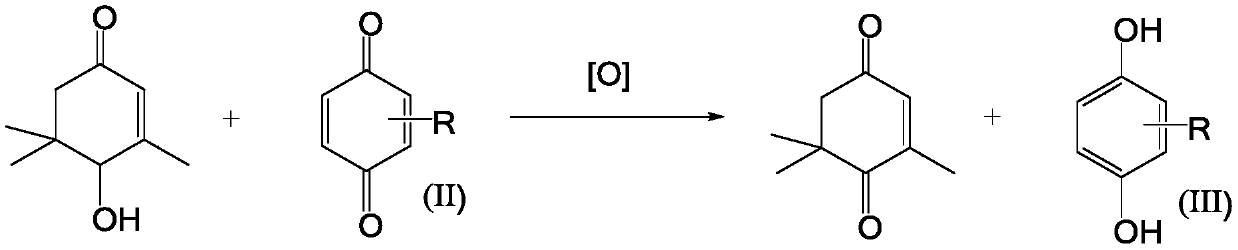
[0049] 1) Add industrial product HIP equivalent to 30.0g (0.1945mol) of pure HIP, 60.0g toluene into a four-neck flask, heat up to 80°C under 300rpm stirring condition, blow air through bubbling method, and add 10.8g p-benzoquinone dropwise (0.10mol) + toluene 30.0g mixed solution, after the dropwise addition, keep the temperature at 80±10°C, continue to ventilate the air, react for 2-5 hours, GC detects that the HIP residue < 0.1% stops the reaction;
[0050] 2) Add aqueous sodium thiosulfate solution (100ml, mass concentration 15-20%) to the reaction solution in step 1) and stir for 30min, then add (in two times, 50mL each time) 5% sodium hydroxide solution for washing, after layering , the aqueous phase is an alkali washing solution, and the organic phase is washed with 30ml of water as a reaction solution,
[0051] 3) In a four-neck flask, stir and feed in air at 70-80°C, react for 2-3 hours, add 30g of toluene and stir for 10min, cool down to room temperature, let stand, ...
Embodiment 2
[0054] 1) Add the industrial product HIP (0.2075mol) equivalent to 32.0g of pure HIP, 60.0g toluene, and heat up to 80°C under 300rpm stirring conditions in a four-necked flask, and add trimethylbenzoquinone dropwise with air by bubbling method 15.0g (0.10mol) + toluene 45.0g mixed solution, after the dropwise addition, keep the temperature at 80±10°C, continue to ventilate the air, react for 3-5 hours, GC detects that the HIP residue < 0.1% stops the reaction;
[0055] 2) Add aqueous sodium thiosulfate solution (100ml, mass concentration 15-20%) to the reaction solution in step 1) and stir for 30min, then add (twice, 70ml each) 5% sodium hydroxide solution for washing, after layering , the aqueous phase is an alkali washing solution, and the organic phase is used as a reaction solution after washing with 50ml of water;
[0056] 3) In a four-neck flask, stir and feed in air at 70-80°C, react for 2-3 hours, add 35g of toluene and stir for 10 minutes, cool down to room temperatu...
Embodiment 3
[0059] 1) Add industrial product HIP equivalent to 35.0g (0.2270mol) of pure HIP, 65.0g p-xylene into a four-necked flask, heat up to 100°C under 300rpm stirring condition, air-bubble method, drop trimethyl Benzoquinone 20.0g (0.1333mol) + p-xylene 45.0g mixed solution, after the dropwise addition, keep the temperature at 100±10°C, continue to ventilate the air, react for 3-5 hours, GC detects that the HIP residue < 0.1% stops the reaction,
[0060] 2) Add aqueous sodium thiosulfate solution (100ml, mass concentration 15-20%) to the reaction solution in step 1) and stir for 30min, then add (twice, 70mL each) 5% sodium hydroxide solution for washing, after layering , the aqueous phase is an alkali washing solution, and the organic phase is washed with 50ml of water as a reaction solution,
[0061] 3) In a four-neck flask, stir and feed in air at 70-80°C, react for 2-3 hours, add 35g of p-xylene and stir for 10min, drop to room temperature, let stand, and separate the organic ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com