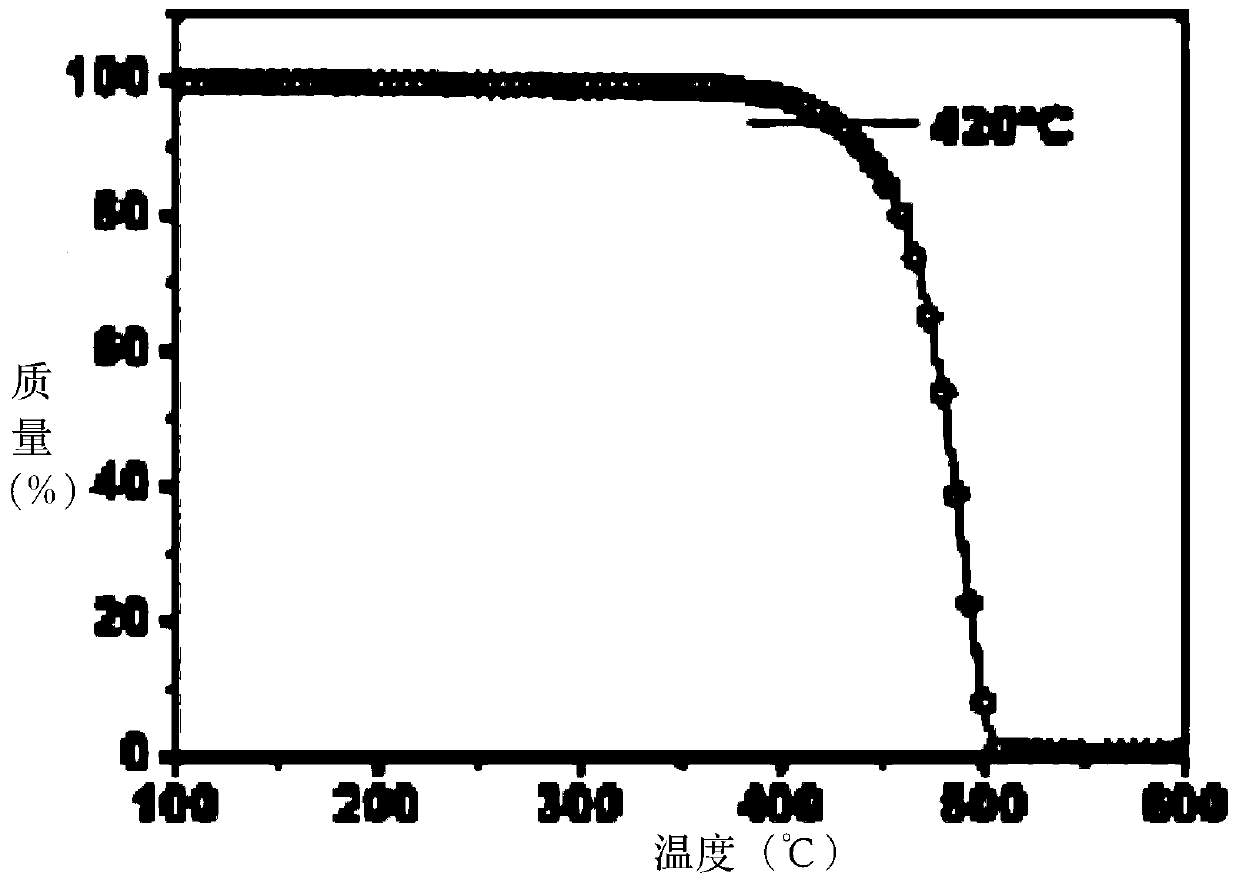
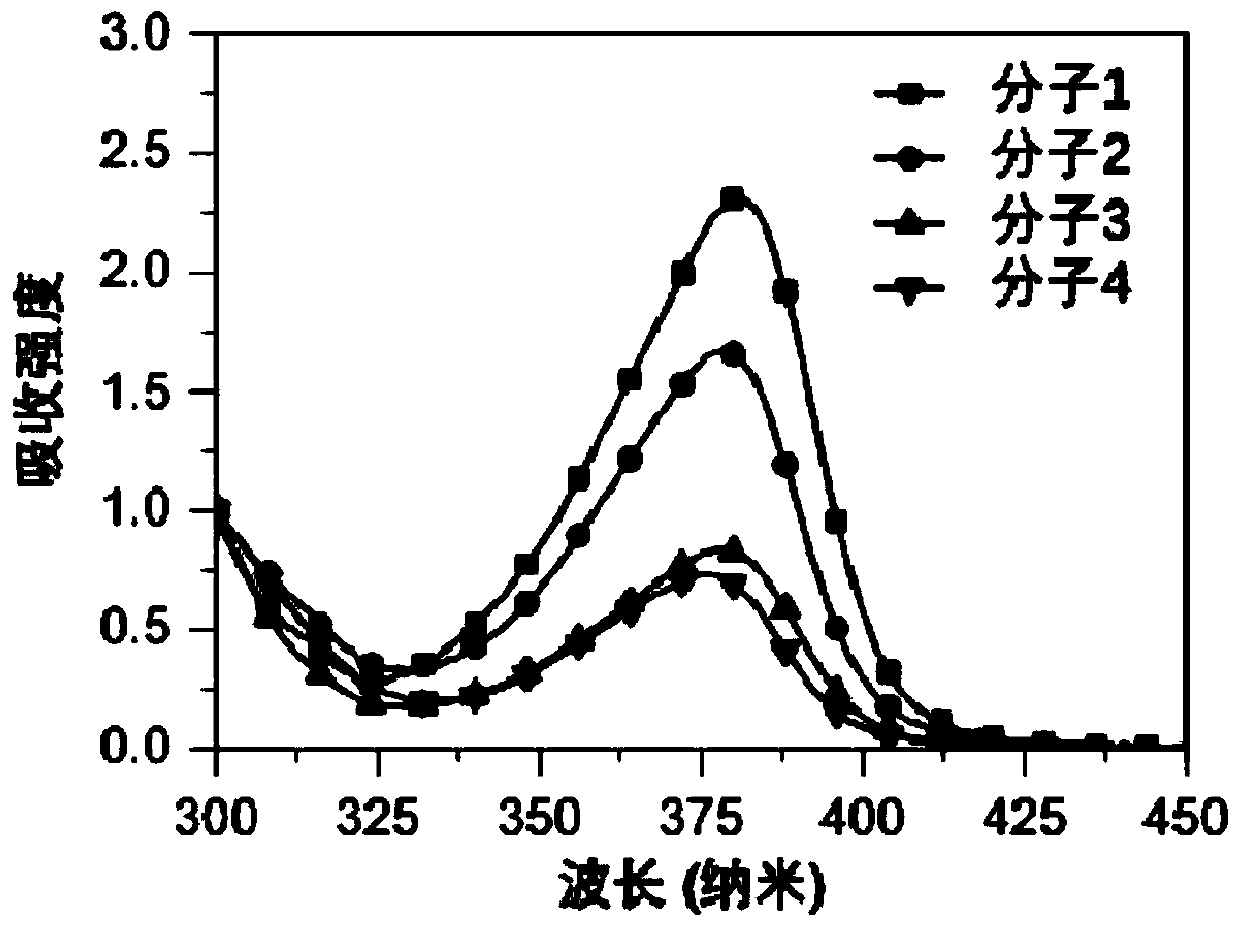
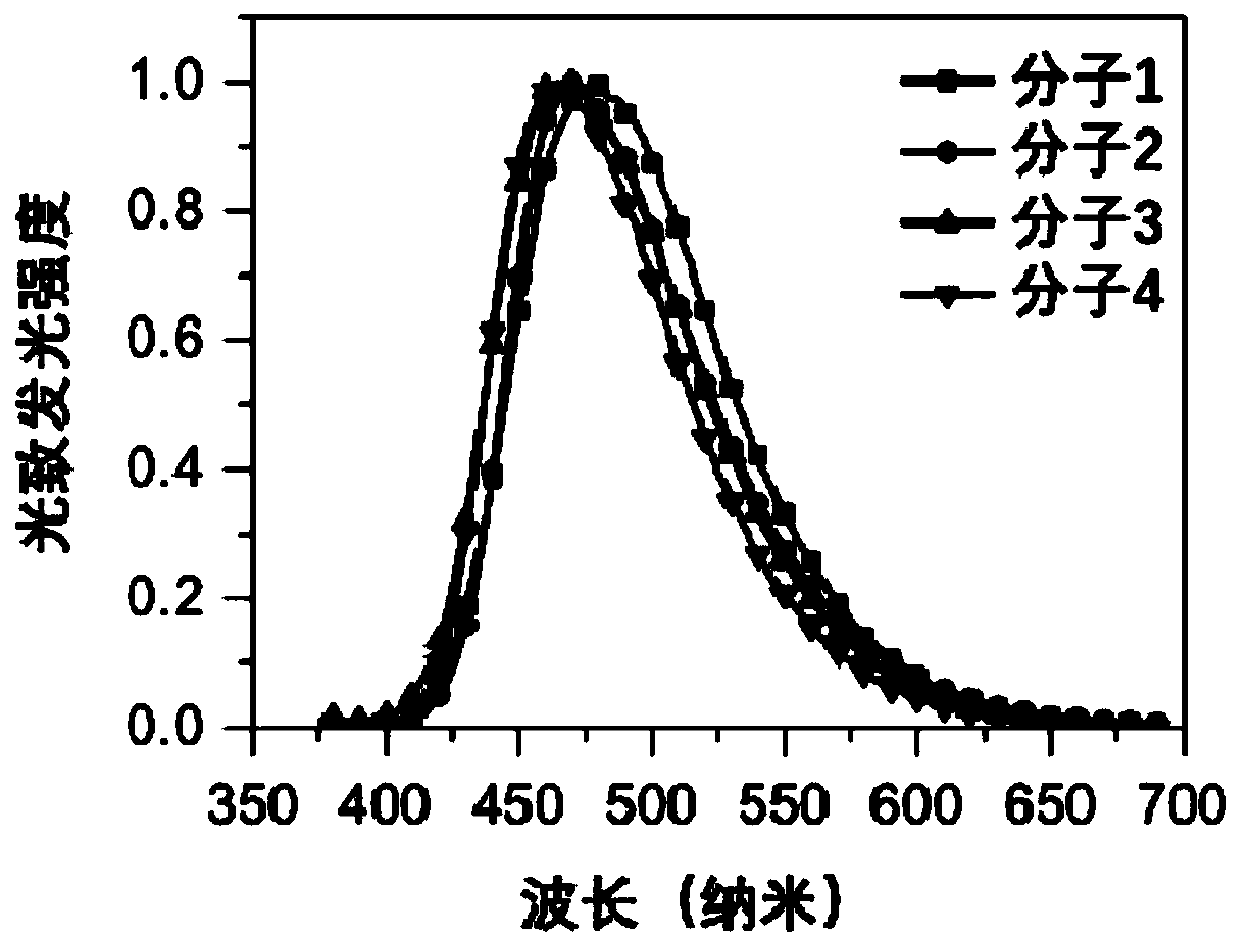
Organic Small Molecule Luminescent Materials and Organic Electroluminescent Devices
A technology of light-emitting materials and small molecules, applied in the fields of light-emitting materials, electro-solid devices, organic chemistry, etc., can solve the problems of limited organic small-molecule optoelectronic materials, and achieve the effect of improving external quantum efficiency, excellent device performance, and high decomposition temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthetic method 1 of 2-bromo-N-phenylaniline, chemical reaction formula is as follows:
[0059]
[0060] In a 250mL three-necked flask, add aniline (0.2mol, 18.6g), o-bromoiodobenzene (0.2mol, 56.58g), palladium acetate (0.6mmol, 134.4mg), sodium tert-butoxide (0.4 mol) into the flask in turn, then add 150mL of toluene, pass N 2 20 minutes, then add tert-butylphosphine (1.2mmol, 1.2ml), continue to pass through N 2 20 minutes, heat to reflux and stir for 12 hours. The temperature was lowered to room temperature, sodium tert-butoxide was removed by suction filtration, the solvent was distilled off under pressure, and the solvent was separated and purified by silica gel chromatography to obtain a colorless oily liquid (27.8 g, yield 56%).
Embodiment 2
[0062] The synthetic method 2 of 2-bromo-N-phenylaniline, chemical reaction formula is as follows:
[0063]
[0064] In a 250mL three-necked flask, add aniline (0.2mol, 18.6g), o-bromoiodobenzene (0.2mol, 56.58g), palladium acetate (0.6mmol, 134.4mg), sodium tert-butoxide (0.4 mol) was added in the flask successively, o-bis(2-phenyl)bis(diphenylphosphine) (0.6mmol, 340mg), then added the toluene of 150mL, logical N 2 20 minutes, heat to reflux and stir for 12 hours. The temperature was lowered to room temperature, sodium tert-butoxide was removed by suction filtration, the solvent was distilled off under pressure, and the solvent was separated and purified by silica gel chromatography to obtain a colorless oily liquid (44.2 g, yield 89%).
[0065] After comparing the above Example 1 and Example 2, it can be found that by changing the catalyst ligand from t-butylphosphine to o-bis(2-phenyl)bis(diphenylphosphine), 2-bromo-N-benzene Aniline yield increased from 56% to 89%. ...
Embodiment 3
[0067] The preparation method 1 of (4-bromophenyl)-phenyl-carbamic acid tert-butyl ester, chemical reaction formula is as follows:
[0068]
[0069] In a 500 ml one-necked flask, di-tert-butyl dicarbonate (BOC) (0.2mol, 43.6g) was added to 400 ml of tetrahydrofuran at room temperature, and then p-N-(dibromophenyl)aniline (0.1mol , 24.8g), heated to reflux and stirred for 24 hours. The mixture was then poured into 1 L of water, and the product was extracted with dichloromethane. Dry the organic phase with anhydrous magnesium sulfate, remove the solvent after separation, and separate and purify with silica gel chromatography to obtain a colorless oily liquid (32.0 g, yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com