Conjugated alkynyl anthracene derivative as well as preparation method and application thereof
A technology for conjugated alkynyl anthracene and derivatives, which is applied in the field of conjugated alkynyl anthracene derivatives, can solve problems such as unfavorable application, isomerization and photooxidation, and achieve the effect of stable photochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
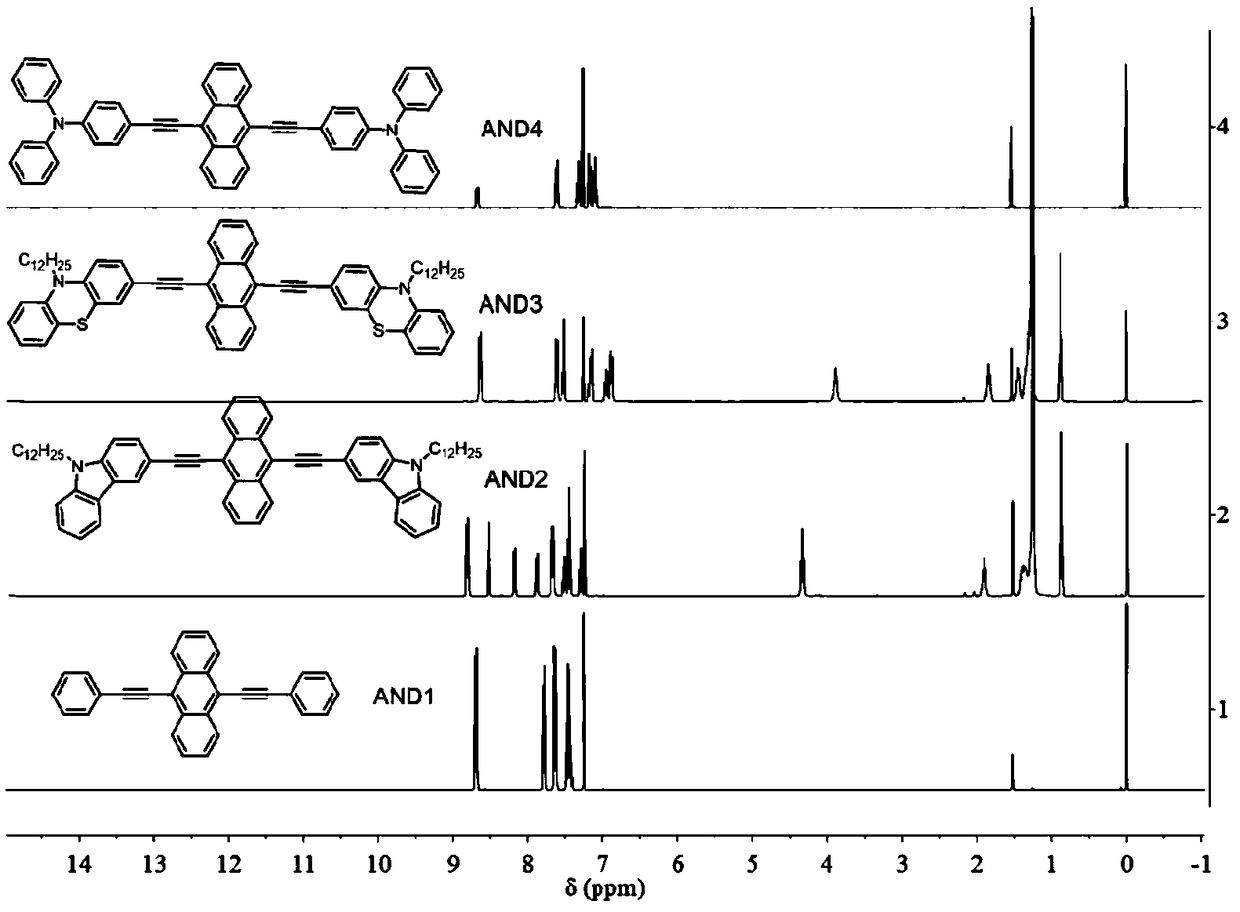
[0030] This embodiment discloses a conjugated alkynyl anthracene derivative, the synthesis method of which is as follows:
[0031]
[0032] Weigh 9,10-dibromoanthracene (1.01g, 3mmol), cuprous iodide (28.5mg, 0.15mmol), triphenylphosphine (39.3mg, 0.15mmol), bis(triphenylphosphine) dichloride Palladium (21.1 mg, 0.01 mmol) was added to a 100 ml three-neck flask equipped with a magnet and a thermometer, and 20 ml of DMF and 15 ml of TEA (triethylamine) were added to dissolve it. Weigh phenylacetylene (0.765g, 7.5mmol), dissolve it with 10ml DMF, and transfer it to a constant pressure dropping funnel. Vacuumize and fill with nitrogen three times under electromagnetic stirring, then raise the temperature to 80°C at 5°C / min, continue stirring for half an hour, and then start to add the DMF solution of phenylacetylene dropwise. TCL monitors that the 9,10-dibromoanthracene raw material reacts completely and then stops heating, and the reaction solution cools down to room tempera...
Embodiment 2
[0036] This embodiment discloses a conjugated alkynyl anthracene derivative, the synthesis method of which is as follows:
[0037]
[0038] Weigh 9,10-dibromoanthracene (1.01g, 3mmol), cuprous iodide (28.5mg, 0.15mmol), triphenylphosphine (39.3mg, 0.15mmol), bis(triphenylphosphine) dichloride Palladium (21.1 mg, 0.01 mmol) was added into a 100 ml three-neck flask equipped with a magnet and a thermometer, and 20 ml of DMF and 15 ml of TEA were added to dissolve it. N-dodecyl-3-ethynylcarbazole (1.43g, 7.5mmol) was weighed, dissolved in 10ml of DMF and transferred to a constant pressure dropping funnel. Under electromagnetic stirring, evacuate and inflate with nitrogen three times, then raise the temperature to 80°C at 5°C / min, continue stirring for half an hour, and start to add the DMF solution of N-dodecyl-3-ethynylcarbazole dropwise. TCL monitors that the 9,10-dibromoanthracene raw material reacts completely and then stops heating, and the reaction solution cools down to...
Embodiment 3
[0043] This embodiment discloses a conjugated alkynyl anthracene derivative, the synthesis method of which is as follows:
[0044]
[0045] Weigh 9,10-dibromoanthracene (1.01g, 3mmol), cuprous iodide (28.5mg, 0.15mmol), triphenylphosphine (39.3mg, 0.15mmol), bis(triphenylphosphine) dichloride Palladium (21.1 mg, 0.01 mmol) was added into a 100 ml three-neck flask equipped with a magnet and a thermometer, and 20 ml of DMF and 15 ml of TEA were added to dissolve it. N-dodecyl-3-ethynylphenothiazine (2.94 g, 7.5 mmol) was weighed, dissolved in 10 ml of DMF and transferred to a constant pressure dropping funnel. Vacuumize and inflate with nitrogen three times under electromagnetic stirring, then raise the temperature to 80°C at 5°C / min, and start to add the DMF solution of N-dodecyl-3-ethynylphenothiazine dropwise after continuous stirring for half an hour. TCL monitors that the 9,10-dibromoanthracene raw material reacts completely and then stops heating, and the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com