RH (Rhein) liposome nanoparticles with kidney targeted distribution characteristics and application
A technology of distribution characteristics and lipid vesicles, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of not being able to effectively improve the efficacy of drugs, and achieve the improvement of multiple pathologies indicators, promote clinical transformation, and improve the effect of therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1 Materials and Instruments
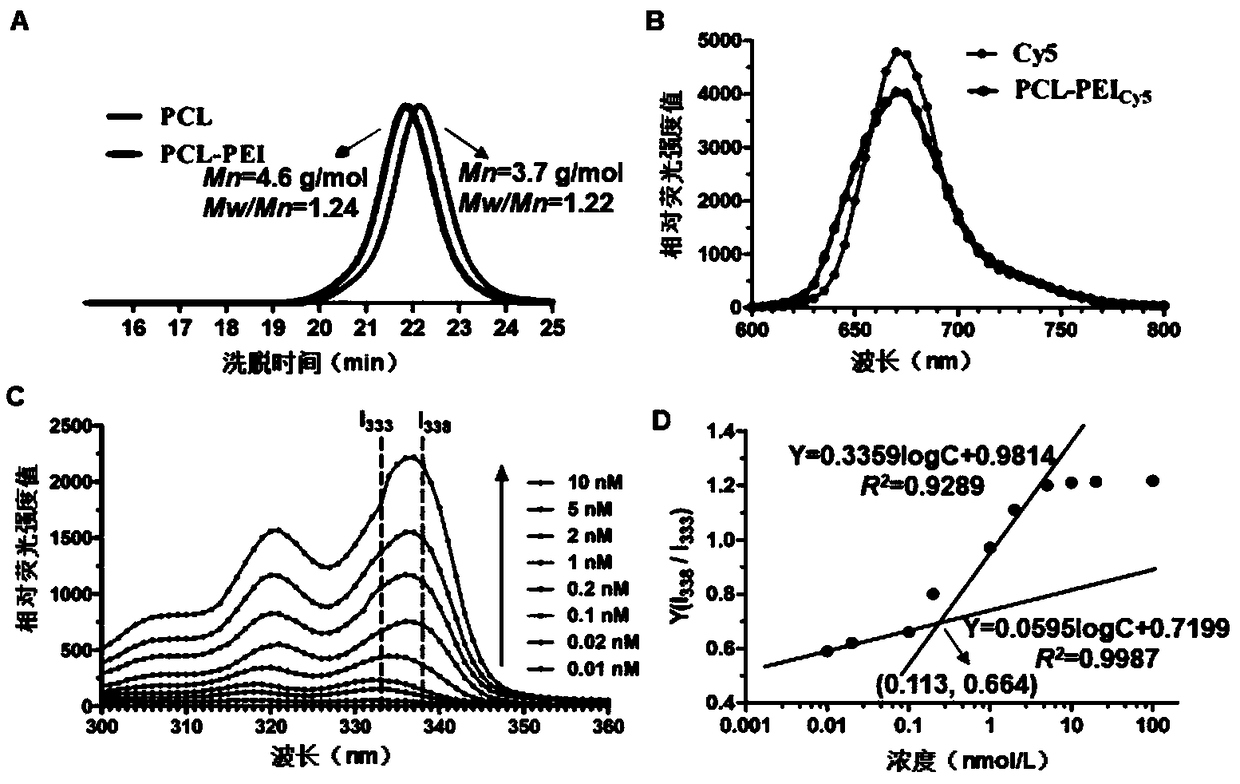
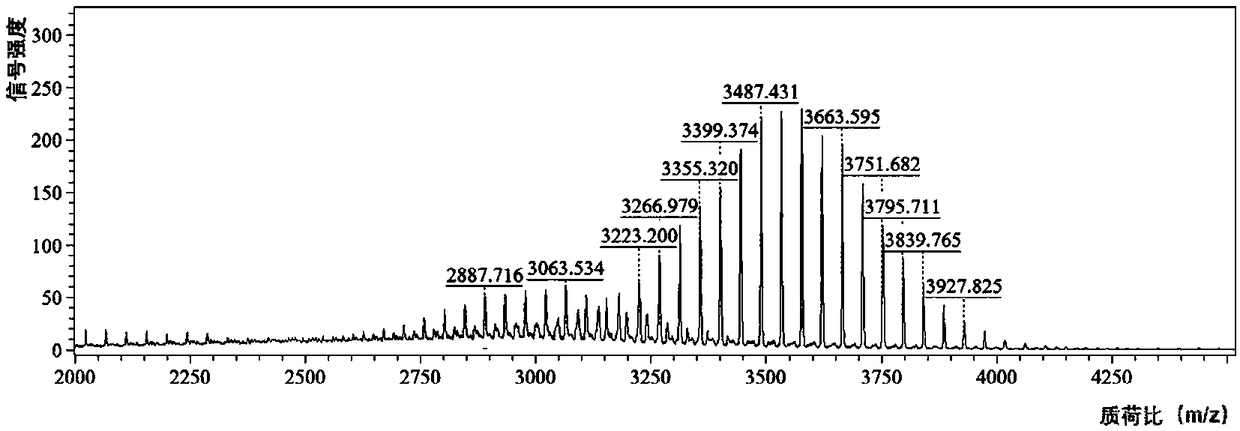
[0031] Kidney Targeting Peptide KCSAVPLC (Kidney Targeting Peptide, KTP, entrusted to Shanghai Qiangyao Biotechnology Co., Ltd. to synthesize, purity ≥ 95%); RH API (Nanjing Zelang Pharmaceutical Technology Co., Ltd., purity ≥ 98%, batch number 201307); RH Reference substance (National Institute for Food and Drug Control, batch number 110757200206); Ethanolamine-polyethylene glycol 2000 (DSPE-PEG) (Shanghai Evert Pharmaceutical Technology Co., Ltd.); 1,2-distearoyl-sn-glycerol-3-phosphoethanolamine-polyethylene glycol 2000-N- Hydroxysuccinimide (DSPE-PEG-NHS) (Nanosoft Polymers, USA); 2-methyl-2-oxazoline, methyl p-toluenesulfonate, 1-3′-dimethylaminopropyl-3 -Ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) (Anaiji Chemical-Saen Chemical Technology Co., Ltd.); carboxyl-terminated polycaprolactone (PCL, molecular weight 3.7kD , Jinan Daigang Bioengineering Co., Ltd.); Thiazole blue (MTT) (Shanghai Aladdin Reagent Co., Ltd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com