Veterinary compound ivermectin injection and preparation method thereof
A technology of ivermectin and injection, which is applied in the field of veterinary compound ivermectin injection and its preparation, can solve the problems of short drug effect maintenance time, labor and material resources, easy to produce drug resistance, etc., and achieve the maintenance of drug resistance. Active, not easy to be infected, and the effect of improving the insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
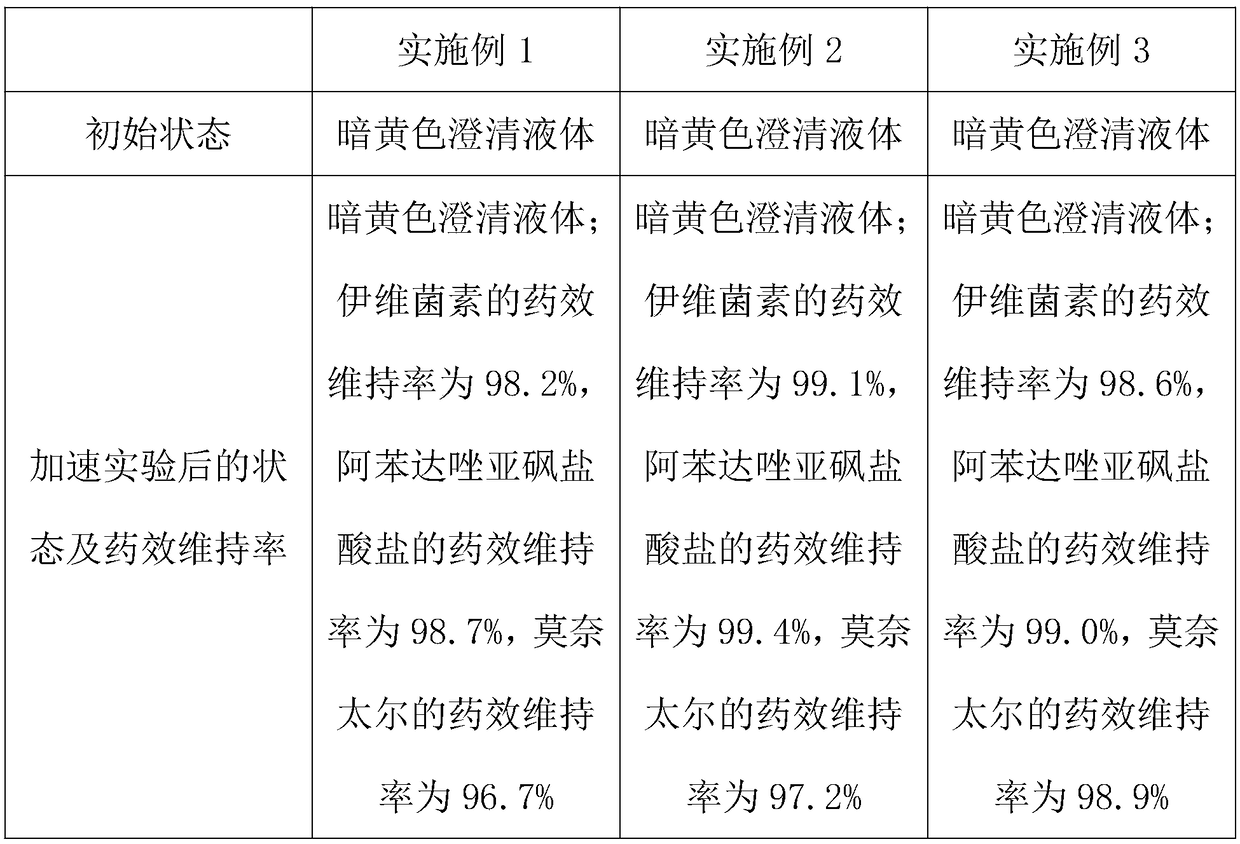
Embodiment 1
[0021] A compound ivermectin injection for veterinary use, the preparation raw materials include 0.5% ivermectin, 1% monepal, 2% albendazole sulfoxide hydrochloride, 0.2% suspending agent, 0.08% emulsifier, 0.01% metal complexing agent, and 96.21% composite solvent;
[0022] Wherein, the suspending agent selects methyl cellulose and Pluronic F-68; the emulsifier selects polysorbate-80; the metal complexing agent selects edetate disodium; The weight ratio is composed of isopropyl myristate:PVP K30:polyethylene glycol:water for injection=2:2:1:4.
Embodiment 2
[0024] A kind of veterinary compound ivermectin injection, the preparation raw material of veterinary compound ivermectin injection comprises 1% ivermectin, 2% monepal, 4.5% albendazole sulfoxide by weight Hydrochloride, 0.5% suspending agent, 0.25% emulsifier, 0.02% metal complexing agent, and 91.73% complex solvent;
[0025] Wherein, the suspending agent is selected from carboxymethyl cellulose and sodium alginate; the emulsifier is selected from lecithin; the metal complexing agent is selected from sodium calcium edetate; Propyl ester: PVP K30: polyethylene glycol: water for injection = 2:2:1:4 composition.
Embodiment 3
[0027] A kind of veterinary compound ivermectin injection, its preparation raw material comprises 1% ivermectin, 3% monepal, 6% albendazole sulfoxide hydrochloride, 0.7% suspending agent, 0.5% emulsifier, 0.04% metal complexing agent, and 88.76% composite solvent;
[0028] Wherein, the suspending agent is selected from polyvinylpyrrolidone, gelatin and sorbitol; the emulsifier is selected from polyoxyethylene castor oil and calcium dodecylbenzenesulfonate; the metal complexing agent is selected from cyclohexanediamine In sodium tetraacetate; the composite solvent is composed of isopropyl myristate: PVP K30: polyethylene glycol: water for injection=2:2:1:4 by weight ratio.
[0029] Above-mentioned each embodiment all adopts following method to prepare injection: step comprises
[0030] S1. Mix and stir isopropyl myristate, PVP K30, polyethylene glycol and water for injection to a clear state to obtain liquid A, and keep it warm at 45°C for later use;
[0031] S2. Disperse or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com