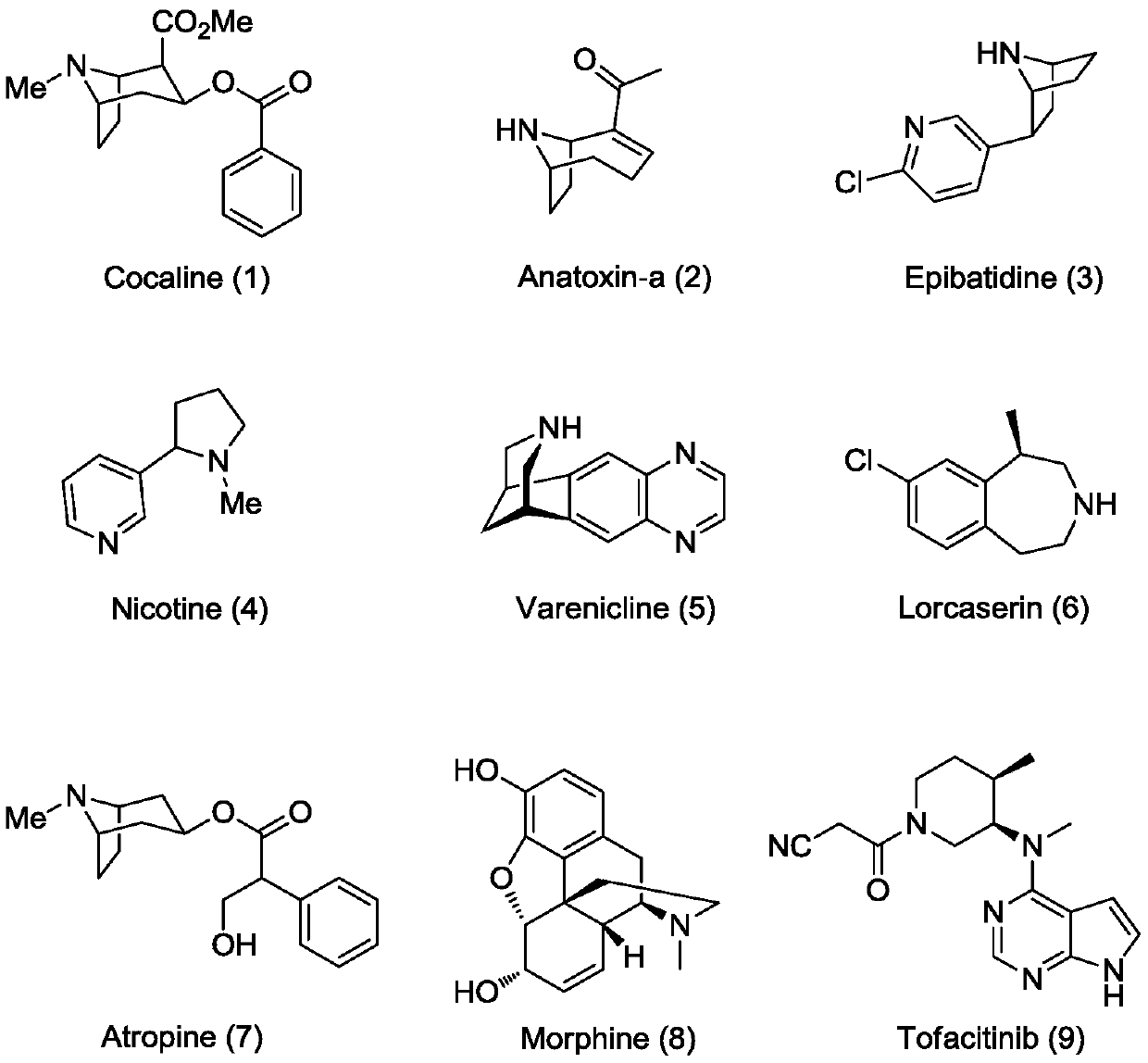
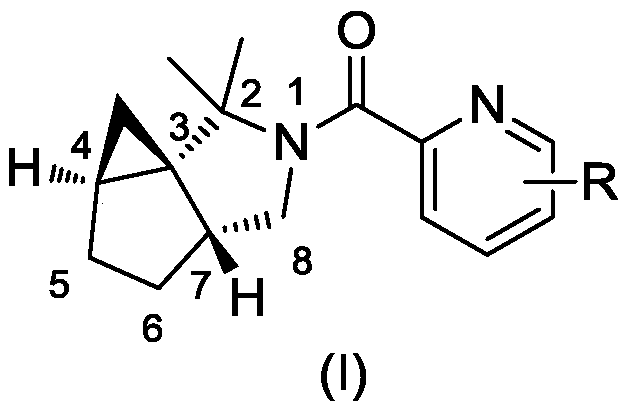
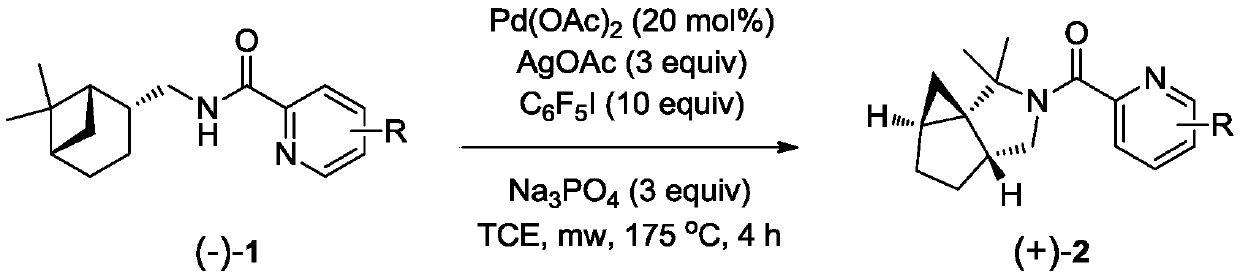A class of chiral azapolycyclic alkaloids and their synthetic methods
A synthetic method and technology of benzene rings, applied in the field of chiral azapolycyclic alkaloids and their synthesis, achieving high site selectivity, high stereoselectivity, and novel reaction mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] (1) Preparation of compound (-)-3:
[0026]
[0027] The procedure was as follows: (-)-cis-Myrtanylamine (680 mg, 4.4 mmol), picolinic acid (652 mg, 5.3 mmol), EDCI (1.26 g, 6.6 mmol), DMAP (54 mg, 0.44 mmol) and dichloromethane (6 mL) Added to a 15mL round bottom flask, stirred and reacted overnight at room temperature. After the reaction was completed, 843 mg of the target product (-)-3 was obtained by separating directly through a silica gel chromatography column (petroleum ether: ethyl acetate = 10: 1), with a yield of 74%. [α] 25 D -8.6 (c 1.76, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.53(d, J=4.4Hz, 1H), 8.19(d, J=7.8Hz, 1H), 8.07(s, 1H), 7.95–7.72(m, 1H), 7.46–7.31(m, 1H ), 3.57–3.37(m,2H),2.41–2.28(m,2H),2.05–1.81(m,5H),1.62–1.50(m,1H), 1.20(s,3H),1.09(s,3H ),0.90(d,J=9.6Hz,1H); 13 C NMR (150MHz, CDCl 3 )δ164.2, 150.0, 148.0, 137.3, 126.0, 122.2, 45.0, 43.7, 41.4, 41.3, 38.7, 33.3, 28.0, 26.0, 23.2, 19.8; HRMS (EI) Calcd for C 16 h 22 N 2 O[M + ]: 2...
Embodiment 1
[0034] The preparation of embodiment 1 compound (+)-4:
[0035]
[0036] The operation is as follows: at room temperature, the pyridinecarboxamide derivative (-)-3 of (-)-cis-Myrtanylamine (ie compound (-)-3) (25.8mg, 0.1mmol), Pd(OAc) 2 (4.5mg, 0.02mmol), AgOAc (50mg, 0.3mmol), C 6 f 5 I (294mg, 1.0mmol), Na 3 PO 4 (49.2mg, 0.3mmol) and TCE (1mL) were added to a 10mL microwave reaction tube with a power of 20W and reacted at 175°C for 4 hours. After the reaction was completed, it was naturally cooled to room temperature, filtered through celite, and spin-dried. Using petroleum ether: ethyl acetate = 4:1 as the developing solvent, 15.7 mg of the target compound (+)-4 was obtained by separation on a preparative plate, with a yield of 61%, [α] 25 D +132.8 (c0.88, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.56(d,J=4.5Hz,1H),7.85–7.71(m,1H),7.67(d,J=7.8Hz,1H),7.30(dd,J=6.8and 5.6Hz,1H), 3.51(dd, J=10.8and 8.0Hz, 1H), 3.31(t, J=10.9Hz, 1H), 2.47(dt, J=10.7and 7.4Hz, 1H), 1.9...
Embodiment 2
[0037] The preparation of embodiment 2 compound (+)-6:
[0038]
[0039] The operation is as follows: at room temperature, the pyridinecarboxamide derivative (-)-5 of (-)-cis-Myrtanylamine (ie compound (-)-5) (33.7mg, 0.1mmol), Pd(OAc) 2 (4.5mg, 0.02mmol), AgOAc (50mg, 0.3mmol), C 6 f 5 I (294mg, 1.0mmol), Na 3 PO 4 (49.2mg, 0.3mmol) and TCE (1mL) were added to a 10mL microwave reaction tube with a power of 20W and reacted at 175°C for 4 hours. After the reaction was completed, it was naturally cooled to room temperature, filtered through celite, and spin-dried. Using petroleum ether: ethyl acetate = 4:1 as a developing solvent, 17.9 mg of the target compound (+)-6 was obtained by separation on a preparative plate, with a yield of 53%, [α] 25 D +70.9(c0.56, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.53(d, J=4.6Hz, 1H), 7.89(d, J=8.1Hz, 1H), 7.17(dd, J=8.1and 4.7Hz, 1H), 3.16(dd, J=9.7and 8.3 Hz,1H),2.85(t,J=10.4Hz,1H),2.52(dt,J=10.6and 7.4Hz,1H),1.90–1.78(m,1H),1.72–1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com