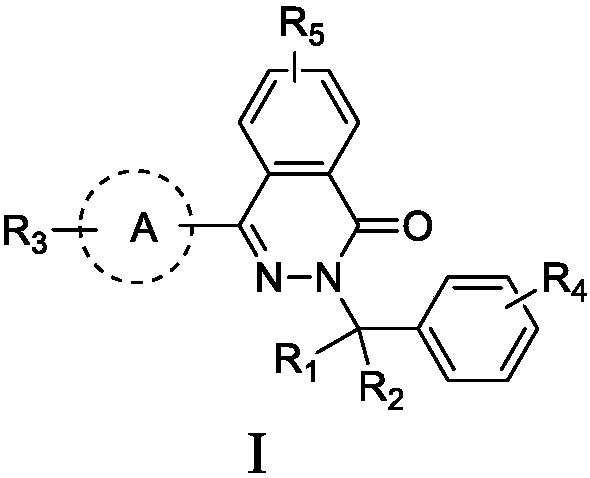
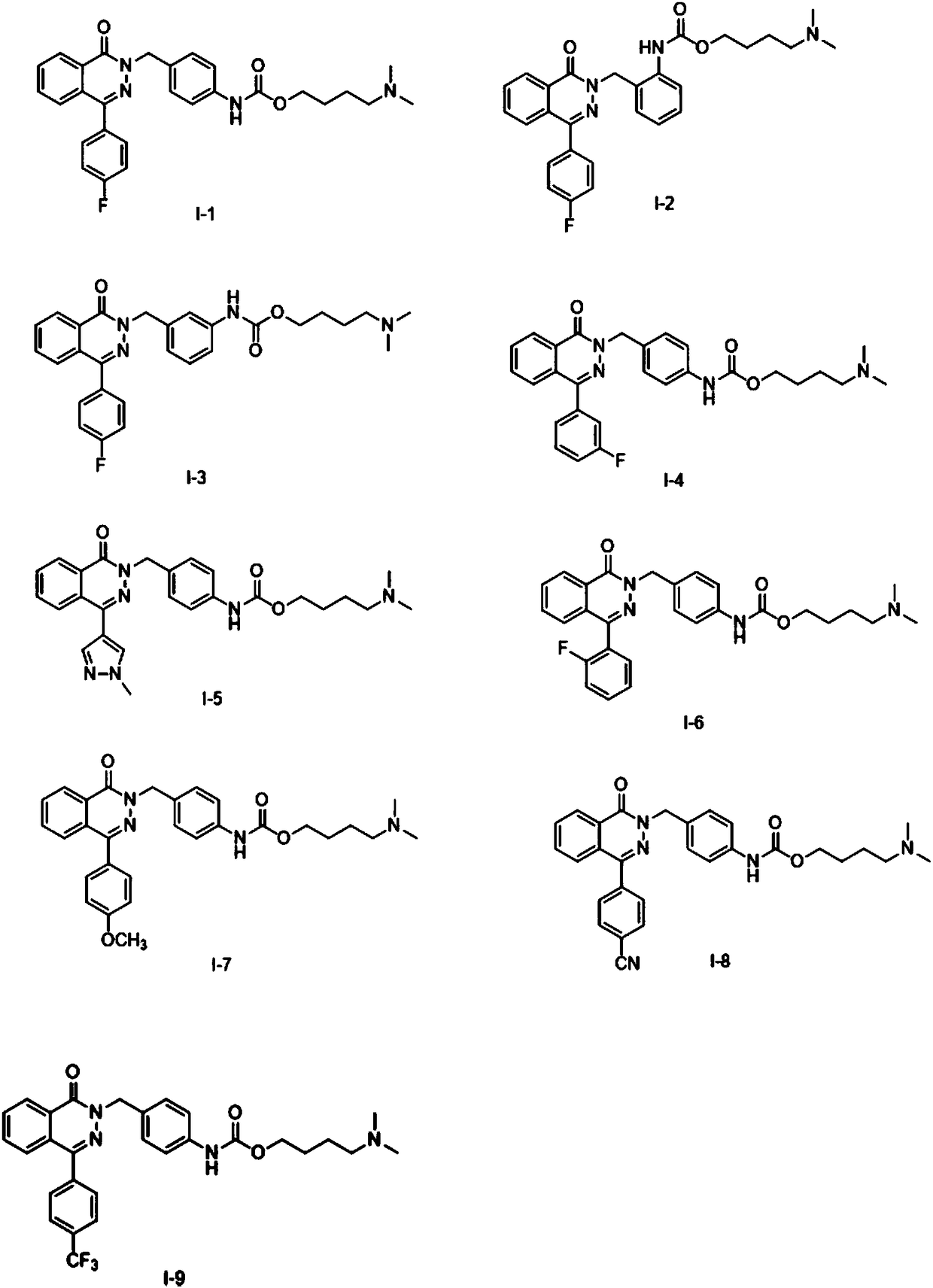
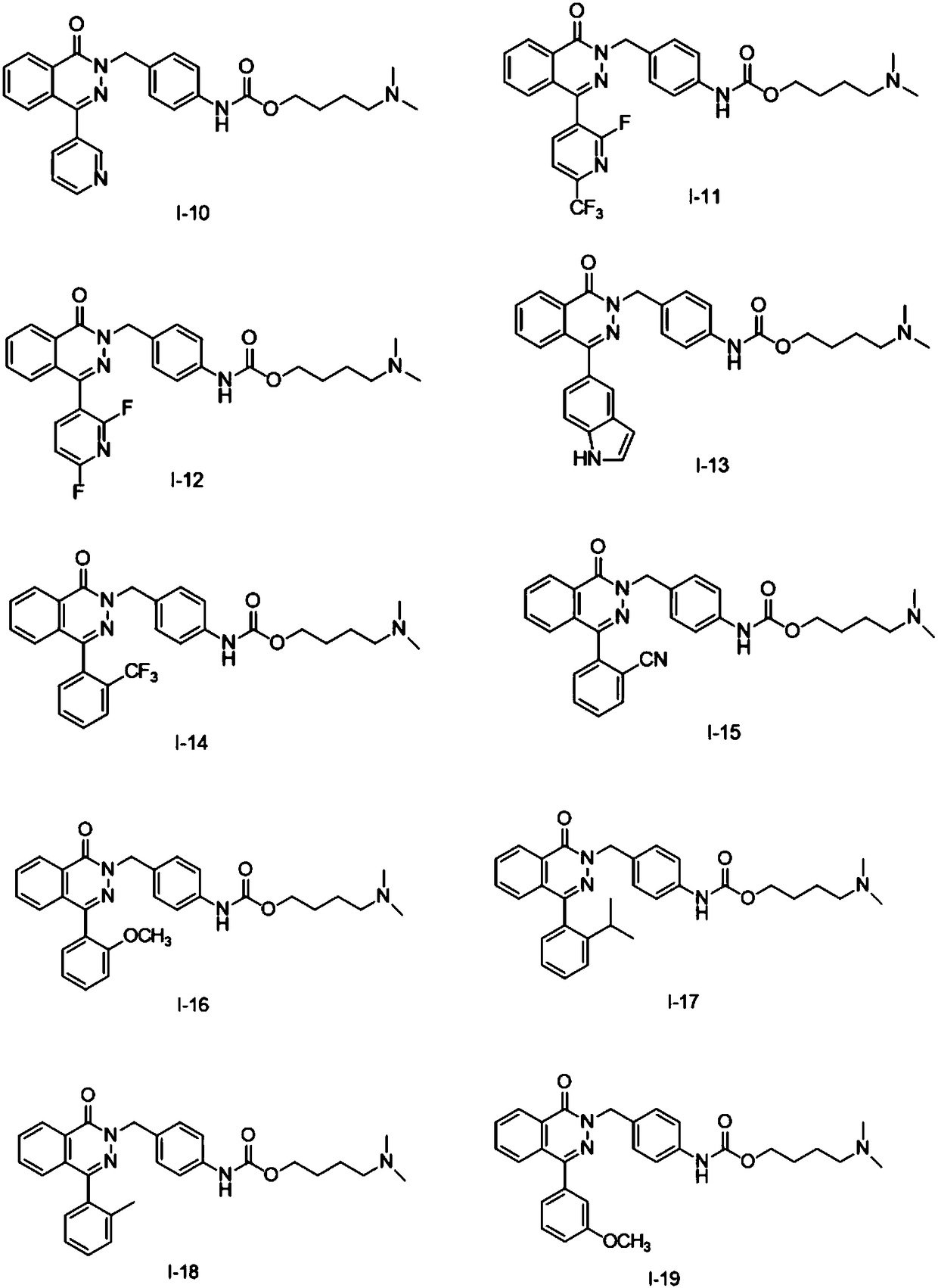
Pyridazinone compound, preparation method thereof, pharmaceutical composition and application thereof
A technology for compounds and solvates, applied in the fields of phthalazinone compounds, their preparation, pharmaceutical compositions and uses, can solve the problems of no marketed drugs, strong variability, dependence on antibody enhancement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1: the preparation of compound I-1
[0093]
[0094] Step 1: Add 10g of 1,4-dichlorophthalazine (compound 1) into 200mL of acetic acid, stir at 120°C for 5h, cool to room temperature, concentrate, wash with water, and filter with suction to obtain 8.5g of white crystals (compound 2) (yield rate of 94%). 1 H NMR (400MHz, CDCl 3 )δ 9.94–9.87 (m, 1H), 8.48 (d, J=8.0Hz, 1H), 8.07 (d, J=8.0Hz, 1H), 8.00–7.87 (m, 2H).
[0095] Step 2: Dissolve 8.5g of compound 2 in 200ml of dioxane and 40ml of water, add 7.21g of p-fluorophenylboronic acid, 1.71g of ferrocene palladium dichloride, 30g of cesium carbonate, nitrogen replacement 3 times, 100 The reaction was overnight at ℃, TLC showed that the substrate disappeared completely, the reaction was stopped and cooled to room temperature, the reaction was extracted with ethyl acetate, the organic layer was washed 3 times, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave 3.6g yellow crys...
Embodiment 2
[0108] Embodiment 2: the preparation of compound II-1
[0109]
[0110] Step 1: Prepare intermediate compound 5 by the same method as in Example 1;
[0111] Step 2: Dissolve 70mg of N,N-dimethylbutyric acid in 20ml of anhydrous dichloromethane, then add 0.15ml of oxalyl chloride and a drop of DMF, react the reaction solution at 0°C for 5-6 hours, stop the reaction and concentrate, and The reaction solution was dissolved in anhydrous THF, and was dropped into 143 mg of the intermediate compound 5 prepared in Example 1 and 0.118 ml of triethylamine THF solution. The reaction solution was reacted overnight at 0° C., TLC monitored the completion of the reaction, and concentrated column chromatography 90 mg of yellow crystals (II-1) were obtained (yield 47%). 1 H NMR (400MHz, CDCl 3 )δ9.86(s,1H),8.51(dd,J=6.8,2.5Hz,1H),7.76(ddt,J=9.8,7.1,3.4Hz,2H),7.69(dt,J=6.5,3.2Hz ,1H),7.56(ddd,J=17.4,8.7,5.3Hz,4H),7.47(d,J=8.5Hz,2H),7.26–7.19(m,2H),5.42(s,2H),2.51( dd,J=14.2,7.3Hz,4H),2....
Embodiment 3
[0116] Embodiment 3: the preparation of compound III-48
[0117]
[0118] Step 1: Dissolve 220mg of chlorophthalazinone in 20ml of N,N-dimethylformamide (DMF), then add 252mg of methyl bromomethylbenzoate and 359mg of cesium carbonate, and react the reaction solution at 50°C for 5 -6 hours, TLC showed that the substrate disappeared completely, stopped the reaction and cooled the reaction solution to room temperature, the reaction solution was extracted with ethyl acetate, the organic layer was washed 3 times, dried with anhydrous sodium sulfate, concentrated, and column chromatography gave 300 mg of yellow Crystal (compound 9) (yield 84.2%)
[0119] Step 2: Dissolve 300mg of intermediate compound 9 in 30ml THF / H2O (5:1), then add 97mg of lithium hydroxide hydrate, and react the reaction solution overnight at room temperature. TLC shows that the substrate completely disappears, and the reaction solution is concentrated. Column chromatography gave 150 mg of yellow crystals (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com