Water-soluble aggregation-induced luminescent probe as well as preparation method and application thereof
An aggregation-induced luminescence and water-soluble technology, which is applied in the field of fluorescent probes, can solve the problems of slow staining speed, detection, and inability to realize fluorescent probes for Aβ plaques, and achieve fast staining and good water-solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
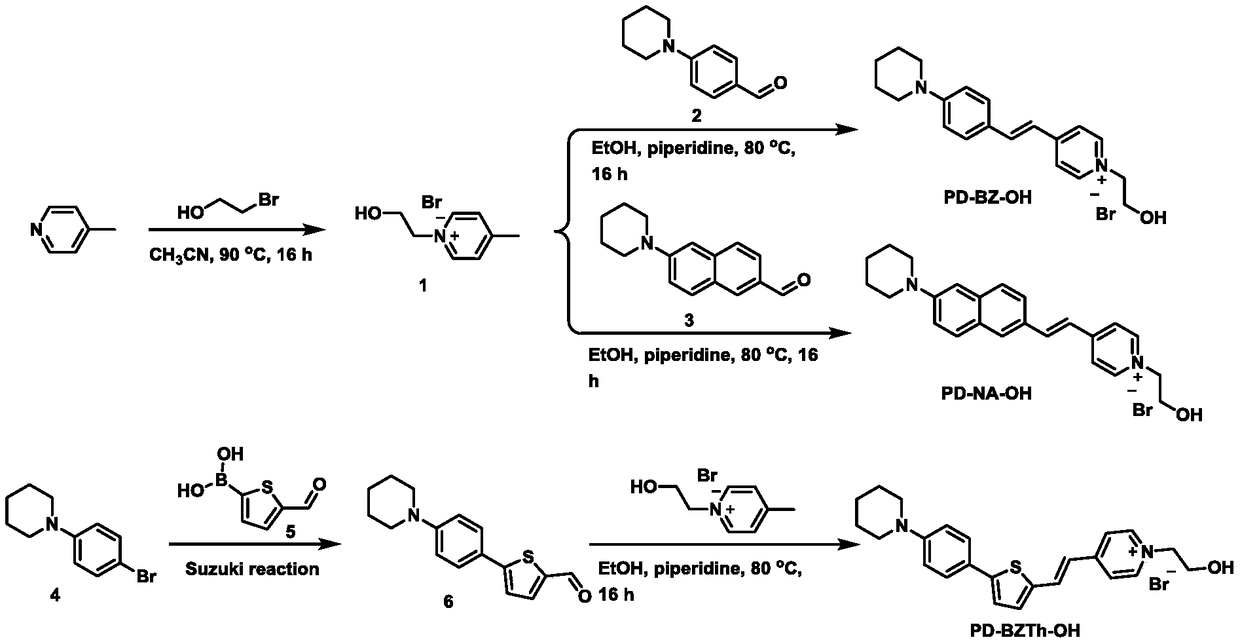
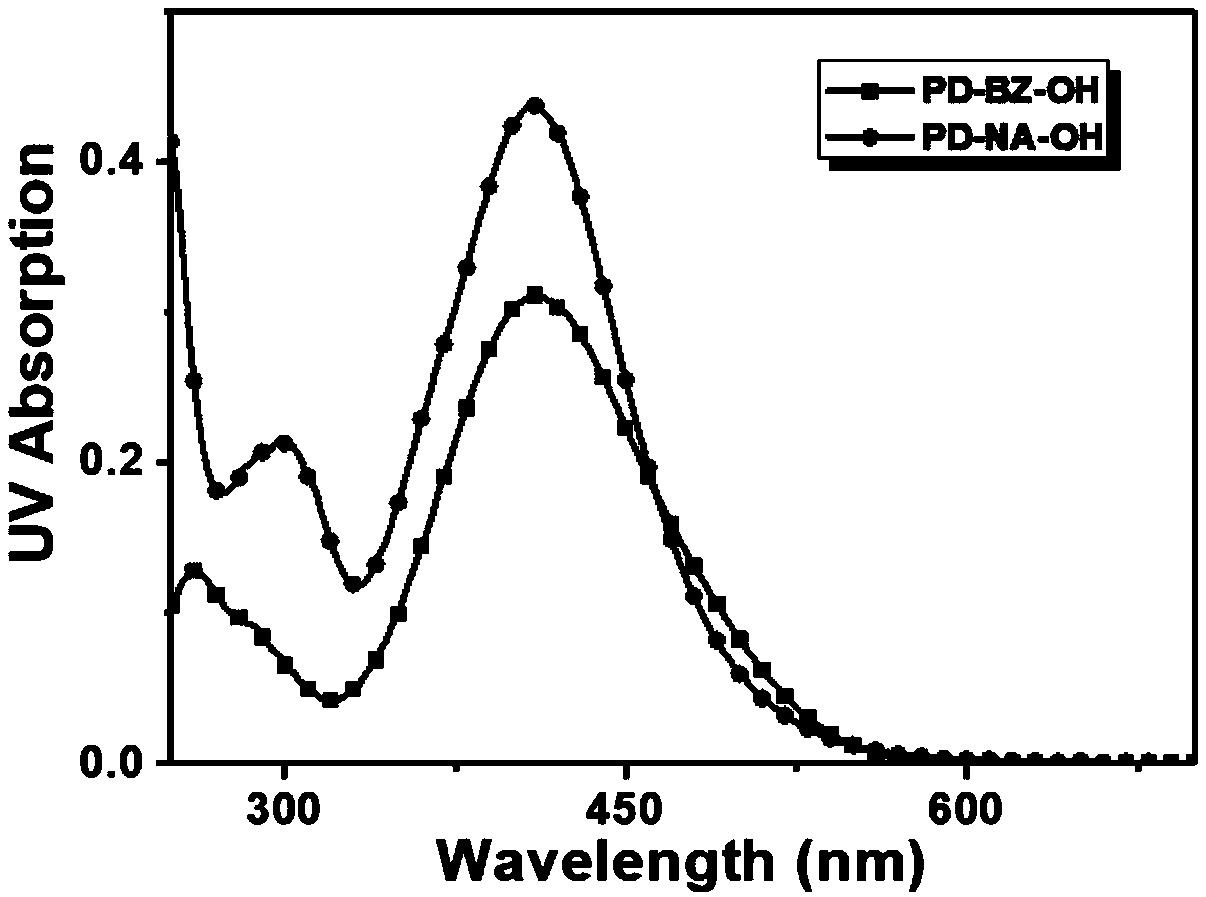
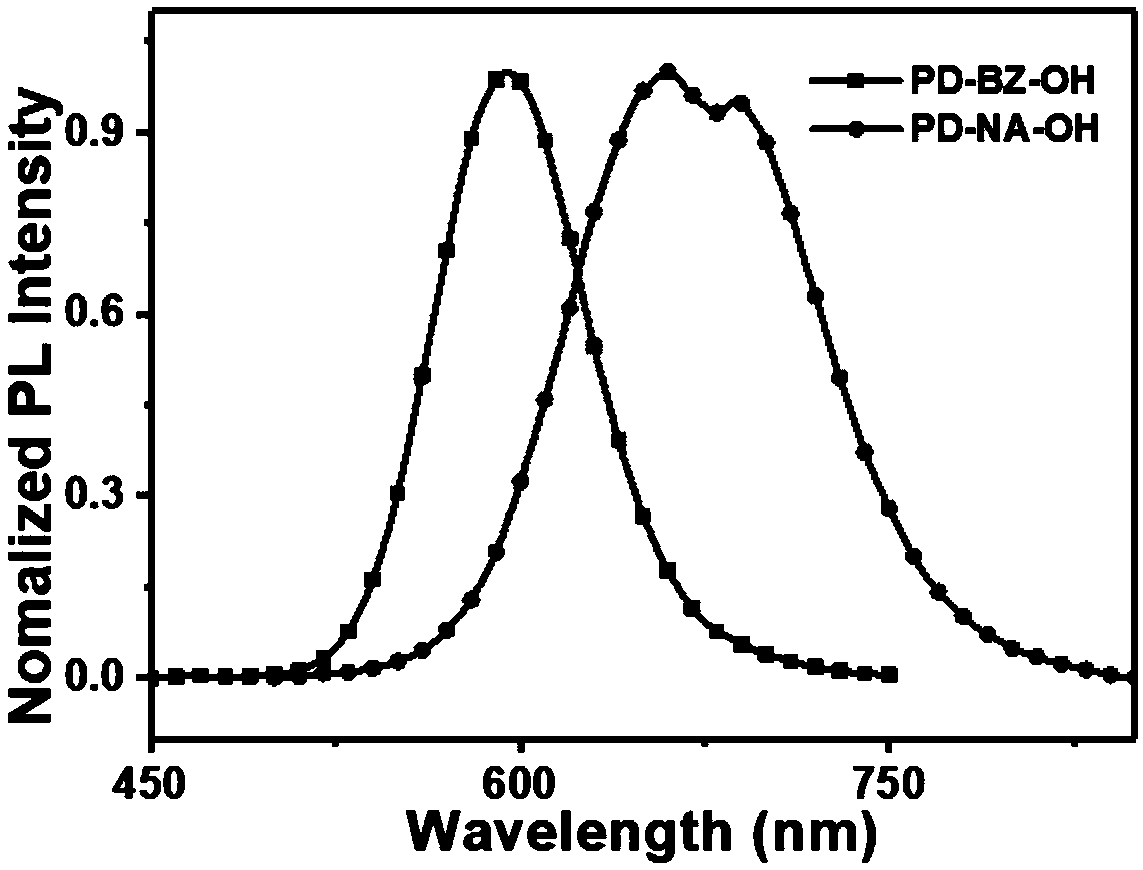
[0036] A fluorescent probe as shown in formula (1), its name is abbreviated as PD-BZ-OH, wherein R 1 for Ar 1 is a benzene ring, Ar 2 for Its synthesis route is as follows figure 1 shown, including the following steps:
[0037] (1) Synthesis of compound 1: 4-picoline (5g, 53.76mmol, 1eq.) and 2-bromoethanol (6.67g, 53.76mmol, 1eq.) were dissolved in CH 3 CN (100 mL) was stirred at 90° C. for 16 hours. Concentration in vacuo afforded compound 1 (11.82 g, crude product) as a yellow oil, which was used in the next reaction without further purification. 1 H NMR (600MHz, DMSO-d 6 )δ8.94(d, J=6.6Hz, 2H), 8.02(d, J=6.6Hz, 2H), 5.17(brs, 1H), 4.67(t, J=4.8Hz, 2H), 3.85(t, J=4.8Hz, 2H), 2.63(s, 3H).
[0038] (2) Synthesis of PD-BZ-OH: ethanol solution of aldehyde 2 (1 eq.), compound 1 (1 eq.) and piperidine (catalytic amount), stirred at 80° C. for 16 hours. After concentration, the crude product was recrystallized from ethanol and ethyl acetate to obtain the title compound...
Embodiment 2
[0040] A fluorescent probe as shown in formula (1), its name is abbreviated as PD-NA-OH, wherein R 1 for Ar 1 is a naphthalene ring, Ar 2 for Its synthesis route is as follows figure 1 shown, including the following steps:
[0041] Synthesis of PD-NA-OH: Aldehyde 3 (1 eq.), Compound 1 (1 eq.) and piperidine (catalytic amount) in ethanol were stirred at 80°C for 16 hours. After concentration, the crude product was recrystallized from ethanol and ethyl acetate to obtain the title compound as a reddish-brown solid. 1 H NMR (600MHz, DMSO-d 6 )δ8.86(d, J=6.6Hz, 2H), 8.24(d, J=6.6Hz, 2H), 8.13(d, J=16.2Hz, 1H), 8.03(s, 1H), 7.83–7.79( m,2H),7.77(d,J=9.0Hz,1H),7.54(d,J=16.2Hz,1H),7.42(dd,J=9.0,2.4Hz,2H),7.20(s,1H), 5.28(t, J=5.3Hz, 1H), 4.56(t, J=5.4Hz, 2H), 3.86(q, J=5.4Hz, 2H), 3.35–3.33(m, 4H), 1.69–1.64(m ,4H),1.63–1.59(m,2H). 13 C NMR (151MHz, DMSO-d 6 )δ153.13,150.31,144.37,141.41,135.68,129.77,129.33,129.21,127.10,126.54,123.79,122.89,121.23,119.28,108.42,61.79,58...
Embodiment 3
[0043] A fluorescent probe as shown in formula (two), its name is abbreviated as PD-BZTh-OH, wherein R 1 for Ar 1 is a benzene ring, Ar 2 for Ar 3 for Its synthesis route is as follows figure 1 shown, including the following steps:
[0044] (1) Synthesis of compound 6: 4-piperidinylbromobenzene (500mg, 2.08mmol, 1eq.) 5-formyl-2-thiophene boronic acid (488mg, 3.12mmol, 1.5eq.), K 2 CO 3 (574mg, 4.16mmol, 2eq.) and Pd(PPh 3 ) 4 (240mg, 0.208mmol, 0.1eq.) and a mixture of toluene / water (20mL / 6mL), at 90°C under N 2 Stir under protection for 16 hours. EtOAc (50 mL) was added thereto, the organic phase was washed with water and brine, washed with Na 2 SO 4 Dry and concentrate in vacuo. The crude product was separated and purified by silica gel chromatography (petroleum ether: DCM=20-70%) to obtain compound 6 as a pale yellow solid (189 mg). 1 H NMR (600MHz, Chloroform-d) δ9.84(s,1H),7.69(d,J=3.9Hz,1H),7.57(s,2H),6.94(d,J=15.2Hz,2H),3.28 (s,4H),1.86–1.58(m,6H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com