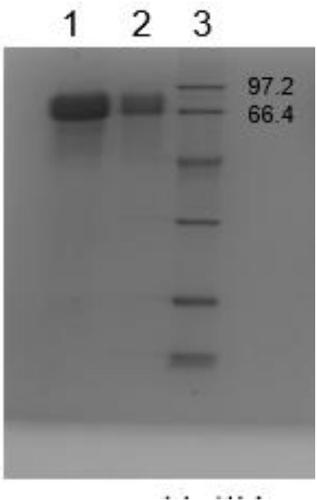
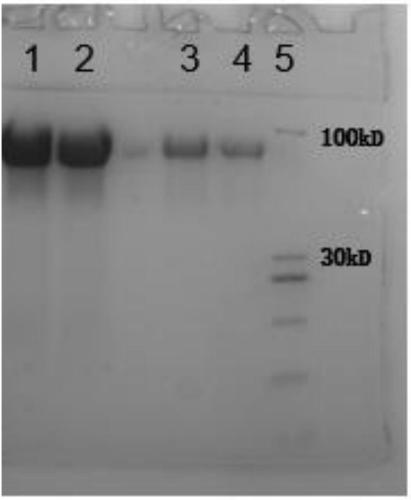
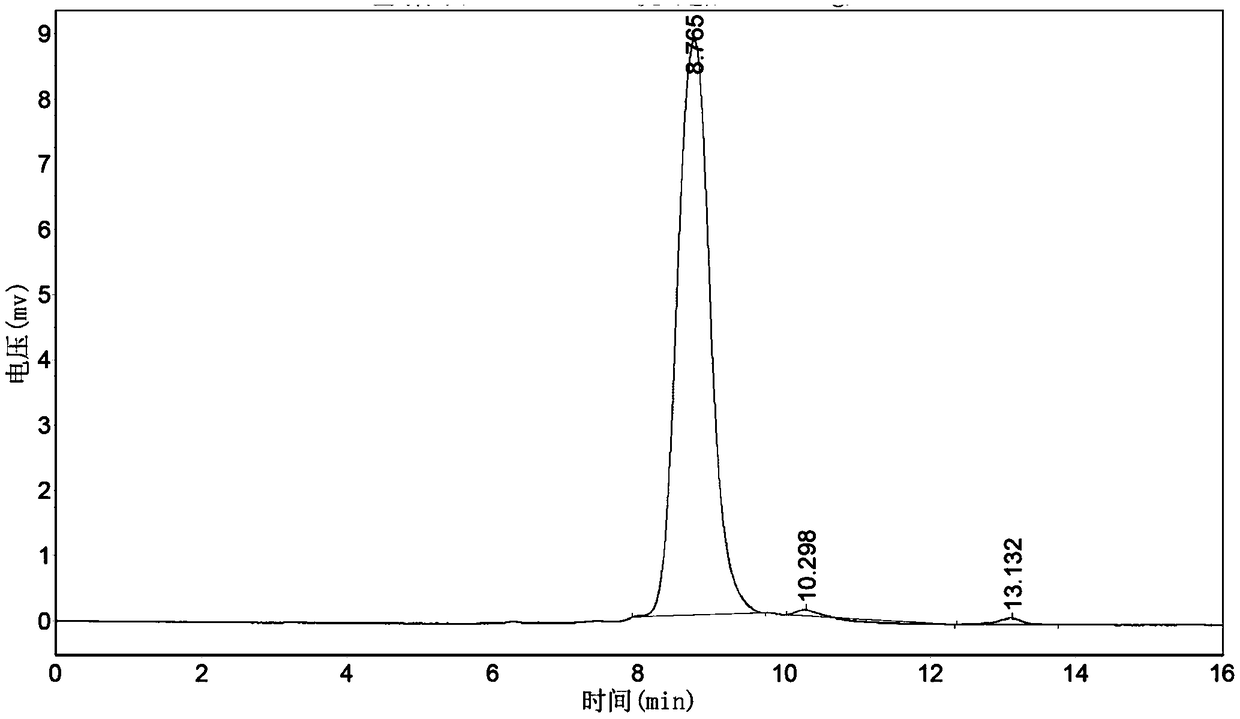
Purification method of natural thrombin regulatory protein
A technology for regulating proteins and purification methods, applied in the fields of peptide preparation, chemical instruments and methods, organic chemistry, etc., can solve the problems of low purification multiple of target protein, complex synthesis process, contamination of target protein, etc. Good purification effect, convenient and quick operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for purifying natural thrombin regulatory protein, comprising the steps of:
[0029] (1) Choose a composite chromatographic column with a hydrophobic group and anisole as a ligand. The composite chromatographic column is a Benzamidine Bestarose 4FF affinity column, and then configure an equilibrium solution and an eluent. The equilibrium solution is 0.05 Mole / liter of phosphate, the eluent includes 0.05 mole / liter of phosphate and 0.5 mole / liter of sodium chloride; the composite chromatography column is balanced with 5 times the column volume of phosphate until the conductivity and pH are consistent with the equilibrium solution , the pH of phosphate is 8.0, and the concentration of phosphate is 0.05 mol / liter;
[0030] (2) Get 500mL concentration of 0.005mg / mL thrombin-regulating protein solution intermediate, adjust the pH of thrombin-regulating protein solution intermediate to 8.0, adjust the conductivity of thrombin-regulating protein solution intermediate ...
Embodiment 2
[0034] A method for purifying natural thrombin regulatory protein, comprising the steps of:
[0035] (1) Choose a composite chromatographic column with a hydrophobic group and anisole as a ligand. The composite chromatographic column is a Benzamidine Bestarose 6FF affinity column, and then configure an equilibrium solution and an eluent. The equilibrium solution is 0.05 mol / L sodium acetate, the eluent includes 0.05 mol / L sodium acetate and 0.3 mol / L sodium chloride; the composite chromatography column is balanced with 3 times the column volume of phosphate until the conductivity and pH are consistent with the equilibrium solution , the pH of phosphate is 5.5, and the concentration of phosphate is 0.05 mol / liter;
[0036] (2) get 200mL concentration and be the thrombin regulatory protein solution intermediate of 10mg / mL, adjust the pH of the thrombin regulatory protein solution intermediate to 5.5, adjust the conductivity of the thrombin regulatory protein solution intermediat...
Embodiment 3
[0040] A method for purifying natural thrombin regulatory protein, comprising the steps of:
[0041] (1) Choose a composite chromatographic column with hydrophobic groups and anisole as a ligand. The composite chromatographic column is a Benzamidine Bestarose 6FF affinity column, and then configure an equilibrium solution and an eluent. The equilibrium solution is 0.02 Mole / liter of sodium acetate, the eluent includes 0.02 mole / liter of sodium acetate and 0.2 mole / liter of sodium chloride; the composite chromatography column is balanced with 5 times the column volume of phosphate until the conductivity and pH are consistent with the equilibrium solution , the pH of phosphate is 4.0, and the concentration of phosphate is 0.02 mol / liter;
[0042] (2) get 500mL concentration and be the thrombin regulatory protein solution intermediate of 10mg / mL, adjust the pH of the thrombin regulatory protein solution intermediate to 4.0, regulate the conductivity of the thrombin regulatory pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com