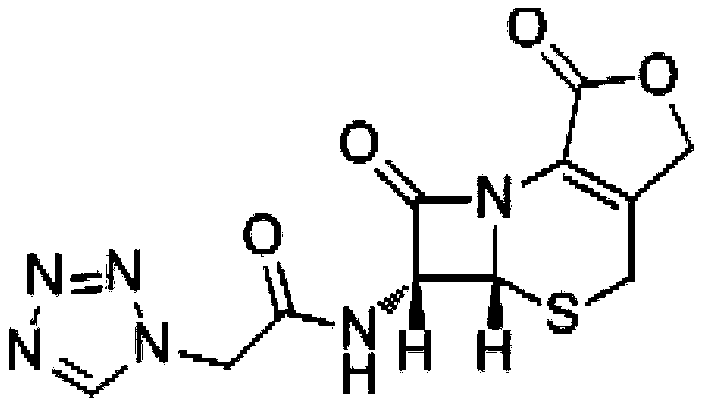
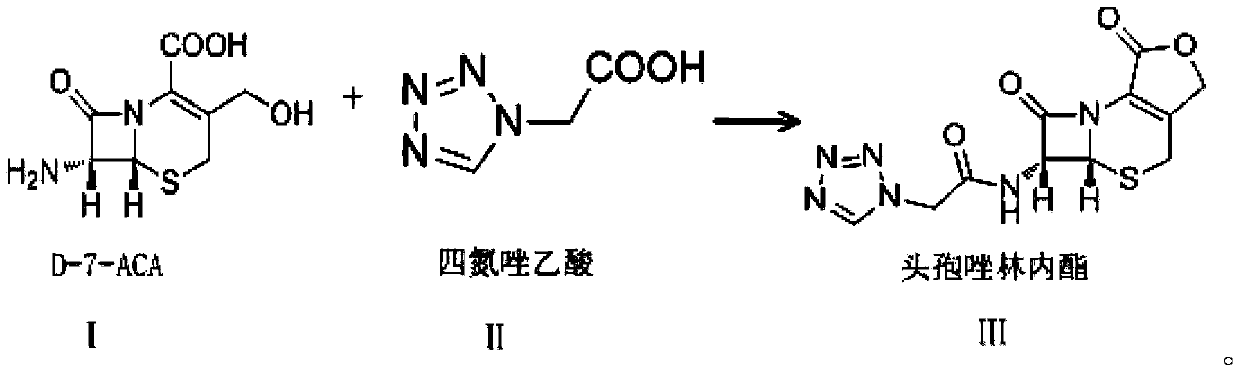
A kind of preparation method of cefazolin lactone
A technology of cefazolin and lactone, which is applied in the field of preparation of cefazolin lactone, can solve problems such as undiscovered, and achieve the effects of easy separation and purification, and simple and fast synthesis operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 15.75g of D-7-ACA and 150mL of dichloromethane into a 500mL four-neck flask, stir for 15 minutes, control the temperature at -5°C to -15°C, add 10.2mL of tetramethylguanidine dropwise, the dropwise addition is completed in about 15 minutes, continue Stir for 30 minutes until D-7-ACA is completely dissolved, cool down to -30°C, and set aside; add 150mL of dichloromethane, 24.74g of tetrazoleacetic acid, 41.3mL of N,N-dimethyl Acetamide, temperature control -10℃~-25℃, stirring for 15min, adding 11mL triethylamine dropwise in about 20min, stirring for another 15min, adding 12mL pivaloyl chloride dropwise in about 30min, stirring for 2h; temperature control at -30℃ Next, add the D-7-ACA solution dropwise into tetrazole acetic acid mixed anhydride, stir for 1 hour, then control the temperature at 10-35°C, add 200 mL of water, adjust the pH to 7.0-7.5 with triethylamine, stir and hydrolyze for 15 minutes , collect the water phase, decolorize with activated carbon, filter,...
Embodiment 2
[0032] Add 15.82g of D-7-ACA and 150mL of dichloromethane into a 500mL four-necked flask, stir for 15 minutes, control the temperature to -15°C to -35°C, add 10.4mL of tetramethylguanidine dropwise, and the dropwise addition is completed in about 15 minutes. Continue to stir for 30 minutes until D-7-ACA is completely dissolved, cool down to -30°C, and set aside; add 150mL of dichloromethane, 25.23g of tetrazoleacetic acid, 41.6mL of N,N-dimethyl Acetamide, temperature control -25℃~-40℃, stirring for 15min, adding 11mL triethylamine dropwise for about 20min, stirring for another 15min, adding 12mL pivaloyl chloride dropwise for about 30min, stirring for 2h; temperature control at -30 Below ℃, add the D-7-ACA solution dropwise into tetrazole acetic acid mixed anhydride, stir for 1 hour, then control the temperature at 10-35℃, add 200mL of water, adjust the pH to 8.0-8.5 with saturated sodium carbonate solution, and stir Hydrolyze for 15 minutes, collect the water phase, decolori...
Embodiment 3
[0034]Add 7.88g of D-7-ACA and 100mL of dichloromethane into a 500mL four-neck flask, stir for 10min, control the temperature to -15°C ~ -35°C, add 5.2mL of tetramethylguanidine dropwise, and the dropwise addition is completed in about 15min. Continue to stir for 30 minutes until D-7-ACA is completely dissolved, cool down to -30°C, and set aside; add 100mL dichloromethane, 12.38g tetrazoleacetic acid, 20.73mL N,N-dimethyl Acetamide, temperature control -10℃~-35℃, stir for 10min, add 5.5mL triethylamine dropwise for about 10min, stir for another 10min, add 6mL pivaloyl chloride dropwise for about 15min, stir for 1h; control temperature at - Below 30°C, add D-7-ACA solution dropwise into tetrazolium acetic acid mixed anhydride, stir for 30 minutes, then control the temperature at 10-35°C, add 100mL of water, adjust the pH to 9.0-9.5 with triethylamine, and stir Hydrolyze for 15 minutes, collect the water phase, decolorize with activated carbon, filter, add 10% hydrochloric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com