Organic second-order nonlinear optical chromophore modified by flexible isolating group and preparation method and application thereof
A second-order nonlinear and chromophore technology, which is applied in the field of organic second-order nonlinear optical chromophores and its preparation, can solve the problems of high nonlinear optical coefficients of chromophores, inability to meet device requirements, and small electro-optical coefficients. , to achieve the effects of increasing intermolecular steric hindrance, easy deviceization, and good film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
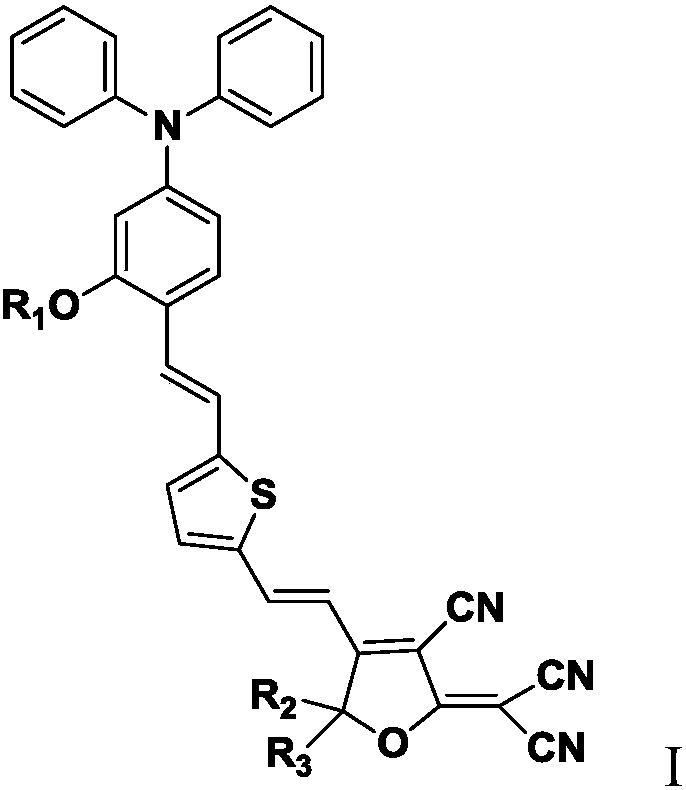
[0071] Preparation of an organic second-order nonlinear optical chromophore CL modified by a flexible isolation group. The structure of CL is as follows:
[0072]
[0073] The preparation route is as follows:
[0074]
[0075] Specific steps are as follows:
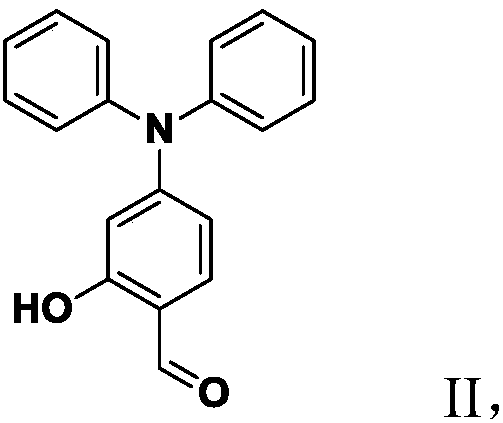
[0076] 1) Compound 1 was synthesized using the method of Enhanced electro-optic activity from thetriarylaminophenyl-based chromosomes by introducing heteroatoms to the donor.
[0077] Add 1.0mL (11mmol) of phosphorus oxychloride dropwise to 5mL N,N-dimethylformamide (DMF) at 0°C. After stirring for 2 hours, slowly add 3.0g (10.91mmol) of 3- Methoxytriphenylamine in 10 mL of DMF. The reaction temperature was raised to 90 °C and the reaction was stirred for 2 h. After cooling, pour the mixed solution into cold water, and add 1mol / l NaHCO 3 The pH of the solution was adjusted until the solution was neutral, and extracted with ethyl acetate, the combined organic phases were washed with anhydrous MgSO 4 Dry overnigh...
Embodiment 2
[0118] A kind of preparation of polymer film, the method is as follows:
[0119] Add 0.075 grams of amorphous polycarbonate, that is, APC, into 1.00 mL of dibromomethane, stir for 3 to 5 hours until APC is completely dissolved, then add 0.025 grams of the organic second-order nonlinear optical chromophore compound CL synthesized in Example 1 to obtain A mixed solution of organic second-order nonlinear optical chromophore compound and APC, the obtained mixed solution is coated on an ITO glass substrate by spin coating, the control speed is 800-1200 rpm, and then dried in a vacuum at 60°C Dry in the box for 24h to obtain the polymer film I. The thickness of the polarized polymer film is between 2-4 μm.
[0120] Determination of polarization and electro-optic coefficient of the synthesized polymer film
[0121] The obtained polymer film I was subjected to corona polarization, the polarization temperature was 165°C, the polarization time was 10-20min, and the polarization voltag...
Embodiment 3
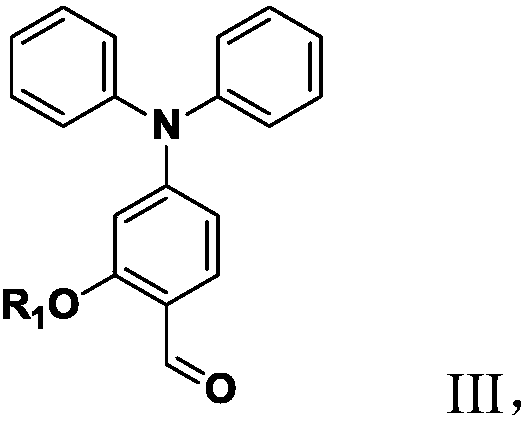
[0123] A preparation of an organic second-order nonlinear optical chromophore modified by a flexible isolation group, the steps are the same as in Example 1, except that:
[0124] Without step 6, the resulting chromophore molecule would look like this:
[0125]
[0126] Compared with Example 1, the flexible spacer group in the organic second-order nonlinear optical chromophore modified by the flexible spacer group in this example is a hexyl hydroxyl group, and the terminal of this group is a hydroxyl group, although its steric effect is not as good as Large silane endcapping appears to be strong, but opens up the possibility for chromophores to be attached in the polymer backbone or side chains using condensation reactions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com