Applications of piceatannol 3-O'-glucoside or piceatannol 3-O'-glucoside derivatives
A technology of tristilbene and its derivatives, which is applied in the field of biopharmaceuticals and can solve problems such as no reports on the use of drugs for the treatment and prevention of ALS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
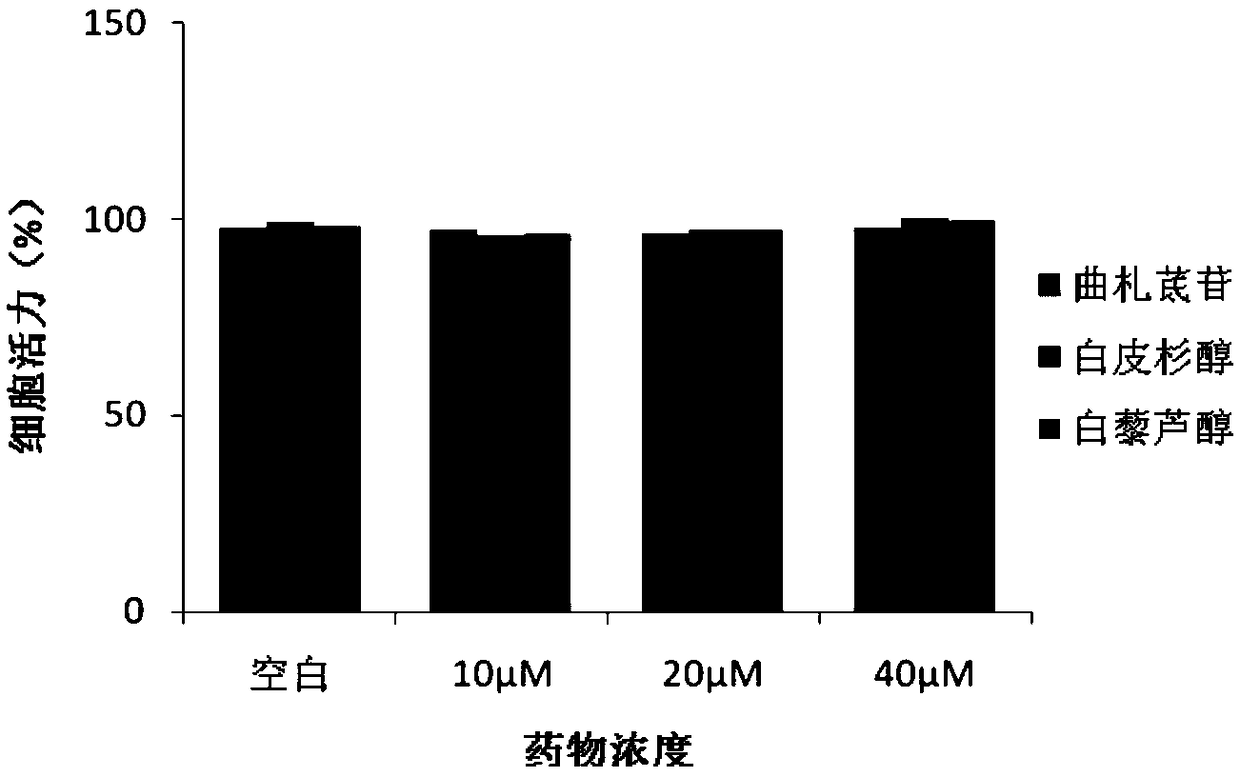
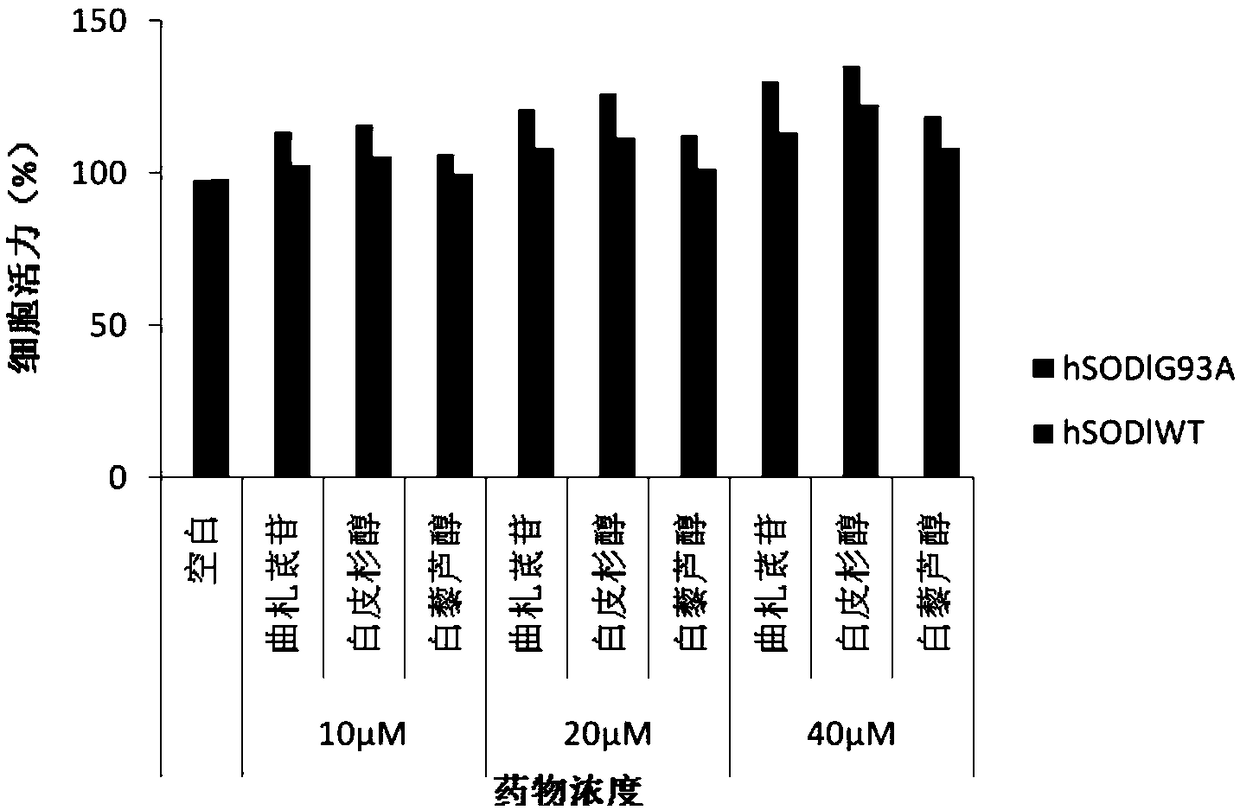
[0034] Effect of Example 1 on Amyotrophic Lateral Sclerosis Cell Model
[0035] 1 test article
[0036] Tristilbene, molecular weight 406, white crystal or crystalline powder, purity 99.6%, batch number 20120402;
[0037] Picetanol, molecular weight 244, reddish-brown powder, purity 99.8%, batch number 906099a;
[0038] Resveratrol, molecular weight 228, white powder, purity 99.8%, batch number CY120325.
[0039] 2 Experimental cells and plasmids
[0040] NSC34 mouse neuron cells were purchased from Guangzhou Geneo Biotechnology Co., Ltd. Cells were cultured in DMEM medium containing 10% FBS at 37°C, 5% CO 2 cultivated in an incubator. Plasmids pF141pAcGFPl SOD1WT and pF145pAcGFPl SOD1G93A were constructed and sequenced by Protin Biotechnology (Beijing) Co., Ltd.
[0041] 3 Reagents and instruments
[0042] DMEM medium, polylysine, DMSO (Gibco, USA); Lipofectamine2000 (Invtrogen, USA); fetal bovine serum (FBS, Hycolne, USA); penicillin-streptomycin (Sigma); ammonium per...
Embodiment 2
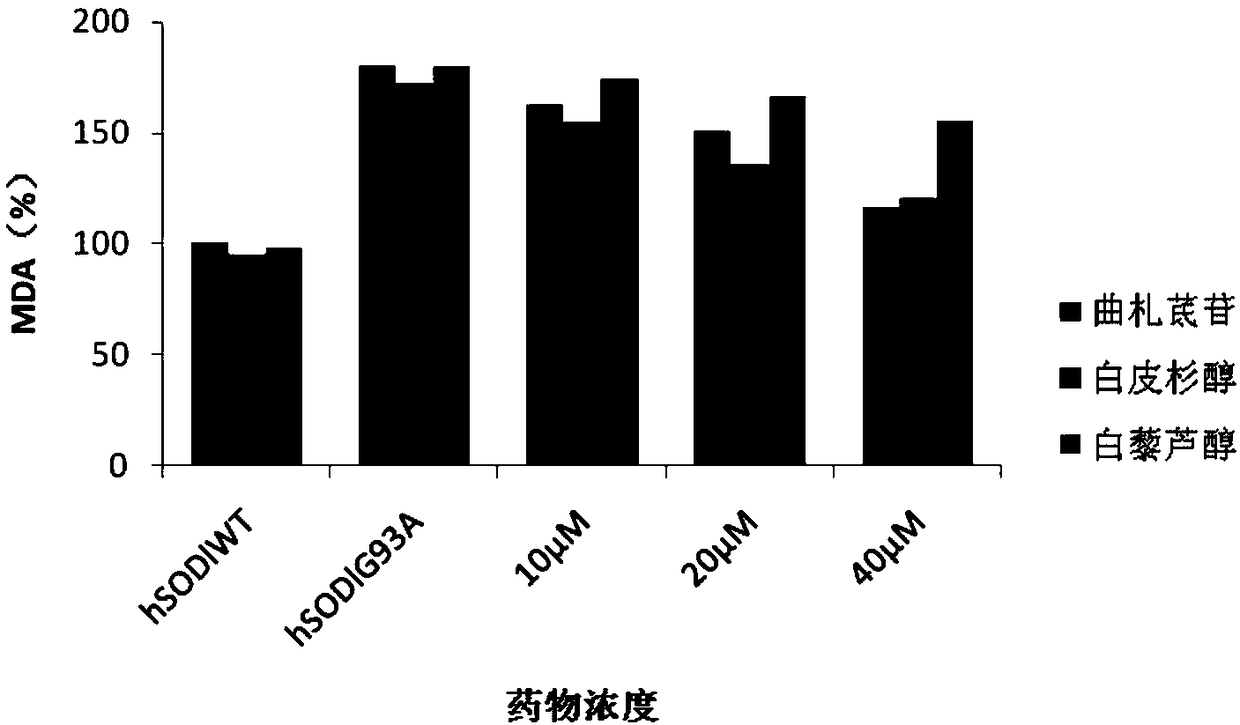
[0061] Example 2 In vitro antioxidant effect
[0062] 1 test article
[0063] Tristilbene, molecular weight 406, white crystal or crystalline powder, purity 99.6%, batch number 20120402;
[0064] Picetanol, molecular weight 244, reddish-brown powder, purity 99.8%, batch number 906099a;
[0065] Resveratrol, molecular weight 228, white powder, purity 99.8%, batch number CY120325.
[0066] The above samples were all provided by the Pharmaceutical Research Institute of Kunming Pharmaceutical Group, sealed at room temperature and kept away from light. During the test, it was weighed with a 1 / 100,000 electronic analytical balance, dissolved, prepared and diluted with DMSO, and the concentration of DMSO in each detection system was controlled at 1%.
[0067] 2 reference substance
[0068] Vitamin C, purity 99.5%, CSPC Weisheng Pharmaceutical (Shijiazhuang) Co., Ltd., batch number 1130172056;
[0069] Edaravone injection, 30mg / 20mL, Kunming Jida Pharmaceutical Co., Ltd., batch n...
Embodiment 3
[0107] Example 3 Effects on Glutamic Acid-Induced Neuroexcitotoxicity
[0108] 1 Experimental materials
[0109] 1.1 Test sample
[0110] Tristilbene, molecular weight 406, white crystal or crystalline powder, purity 99.6%, batch number 20120402;
[0111] Picetanol, molecular weight 244, reddish-brown powder, purity 99.8%, batch number 906099a;
[0112] Resveratrol, molecular weight 228, white powder, purity 99.8%, batch number CY120325.
[0113] Preparation method: The test substance is first prepared into 10mM stock solution with DMSO, and then diluted with normal saline to the required concentration.
[0114] 1.2 Negative control substance
[0115] Sodium chloride injection (0.9% normal saline), Qingzhou Yaowang Pharmaceutical Co., Ltd., batch number: 2215110901, specification: 500ml / bottle.
[0116] 1.3 Positive control substance
[0117] Edaravone injection, Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd., batch number: 80-170102, specification: 5ml: 10mg.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com