Benzophenone type derivative photoinitiator and preparation method thereof
A technology of benzophenone and photoinitiator is applied in the field of benzophenone derivative photoinitiator and its preparation, which can solve the problems of short absorption wavelength of photoinitiator, and achieves improvement of short absorption wavelength and reaction Mild conditions and enhanced light absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
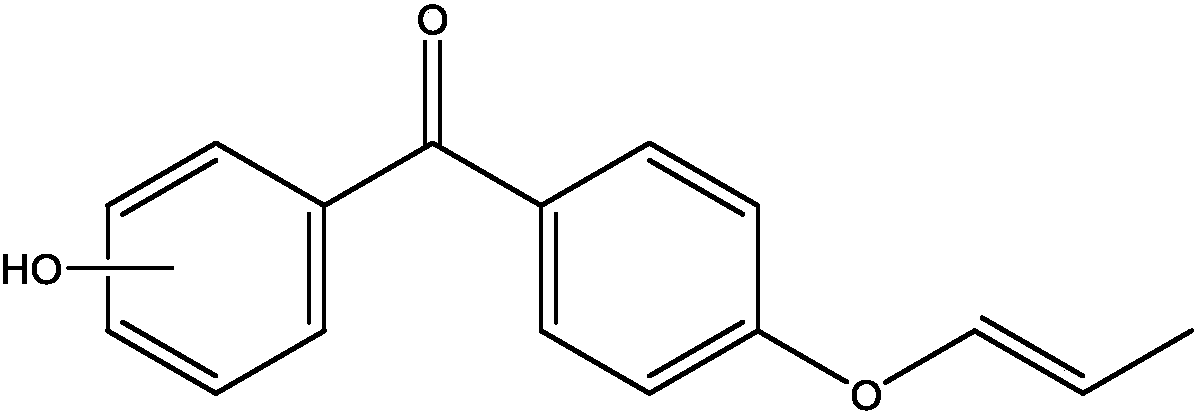
[0027] The structural formula of compound 1 is:
[0028]
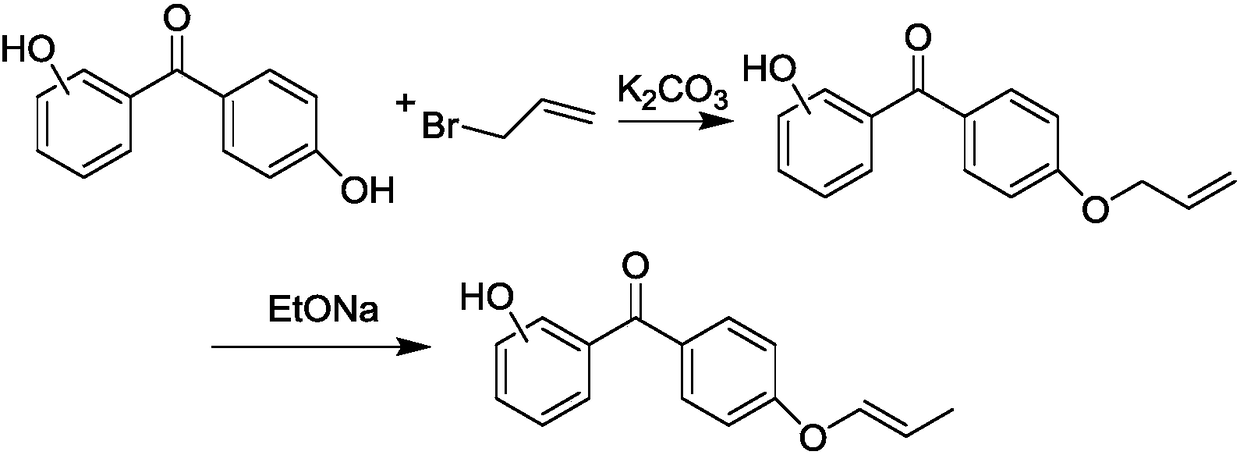
[0029] 9.91g (50mmol) of 4-hydroxybenzophenone, 3.46g (25mmol) of potassium carbonate, and 80ml of carbon tetrachloride were added together into a 250ml three-necked bottle equipped with a magnetic stirrer and a thermometer, and heated and stirred with nitrogen gas. Mix 9.25g (75mmol) of 3-bromopropene and 154g (1mol) of carbon tetrachloride in a constant pressure funnel, then slowly add the mixed solution into a three-necked flask, heat up to 60°C and reflux for 24h. After the reaction, Remove solid impurities by filtration, wash with water and separate liquid for 3 times to remove salts, remove the solvent by rotary evaporation, and then recrystallize to obtain white powder crystals, 4.76g.
[0030] Add 4.76g (about 0.02mol) of the white powder obtained above and 100ml of absolute ethanol (dried to remove water) into a 250ml three-necked bottle equipped with a reflux condenser and a magnetic stirrer, blow nitrogen...
Embodiment 2
[0033] The structural formula of compound 2
[0034]
[0035] Add 9.91g (50mmol) of 4,4'-dihydroxybenzophenone, 3.46g (25mmol) of potassium carbonate, and 100ml of acetone into a 250ml three-necked bottle equipped with a magnetic stirrer and a thermometer, blow nitrogen gas, and heat and stir. Mix 6.15g (51mmol) of 3-bromopropene and 60g (1mol) of acetone in a constant pressure funnel, and then slowly add the mixed solution into a three-necked flask, heat up to 60°C and reflux for 24h. After the reaction, filter to remove the solid Impurities were washed with water and separated three times to remove salts, and the solvent was removed by rotary evaporation, and recrystallized to obtain white powder crystals, 5.13 g.
[0036] Add 5.13g (about 0.02mol) of the white powder obtained above and 100ml of absolute ethanol (dried to remove water) into a 250ml three-necked bottle equipped with a reflux condenser and a magnetic stirrer, blow nitrogen gas, stir for about 1h, and remove...
Embodiment 3
[0039] The structural formula of compound 3 is:
[0040]
[0041] 9.91g (50mmol) of 4-hydroxybenzophenone, 7.92g (50mmol) of potassium carbonate, and 120ml of carbon tetrachloride were added together into a 250ml three-necked bottle equipped with a magnetic stirrer and a thermometer, and heated and stirred with nitrogen gas. Mix 12.30 g (100 mmol) of 3-bromopropene and 144 g (2 mol) of tetrahydrofuran in a constant pressure funnel, then slowly add the mixture into a three-necked flask, heat up to 60°C and reflux for 24 hours. After the reaction is complete, filter to remove the solid Impurities were washed with water and liquid-separated three times to remove salts, and the solvent was removed by rotary evaporation, and recrystallized to obtain white powder crystals, 5.78g.
[0042] Add 5.78g (about 0.02mol) of the white powder obtained above and 100ml of absolute ethanol (dried to remove water) into a 250ml three-necked bottle equipped with a reflux condenser and a magneti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com