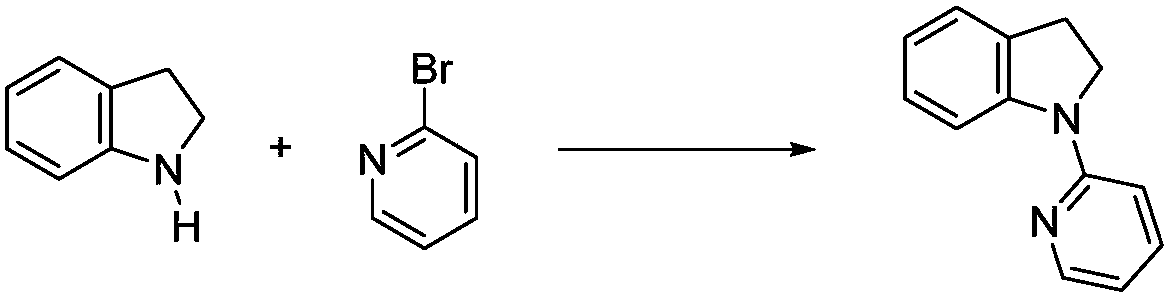
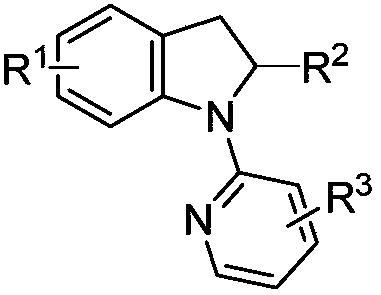
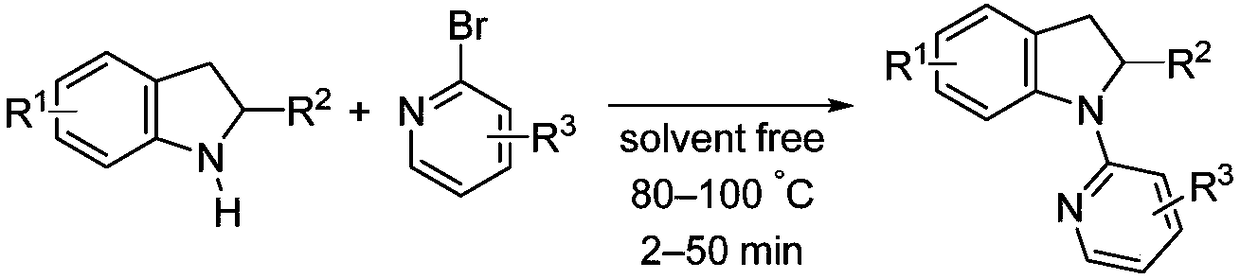
Solvent-free synthesis method of 1-(2-pyridyl)indoline derivative
A synthetic method, indoline technology, applied in the direction of organic chemistry, etc., to achieve the effect of mild conditions, good atom economy, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] This synthetic method specifically comprises the following steps:
[0029] Step 1: Add indoline or indoline derivatives and pyridine derivatives into corresponding round bottom flasks; the pyridine derivatives are 2-chloropyridine derivatives or 2-bromopyridine derivatives.
[0030] Step 2: The ratio of the amount of substances between the indoline derivatives and the 2-chloropyridine derivatives is 1:1 to 1:1.5; the ratio of the amounts of the substances between the indoline derivatives and the 2-bromopyridine derivatives is 1 : 1~1: 1.5;
[0031] Step 3: Stir directly in the air, mix evenly at room temperature, then turn on the heating, set the temperature at 80-100°C, stop the reaction after the system solidifies, and cool to room temperature;
[0032] Step 4: Add a certain amount of saturated NaHCO to the system 3 Solution, dissolve the solid, then add an appropriate amount of ethyl acetate solution, shake, separate the liquid, extract the aqueous phase with ethyl...
Embodiment 1
[0037]
[0038] A kind of synthesis of 1-(2-pyridyl) indoline:
[0039] ① Add 1.19g of indoline and 1.73g of 2-bromopyridine into a 50mL round bottom flask, and the ratio of the substances is 1: 1.1;
[0040] ② Stir directly in the air, mix evenly at room temperature, then heat to 100°C, the system solidifies after 2 minutes, stops the reaction, and cools to room temperature;
[0041] ③Add 50mL saturated NaHCO to the system 3 solution, dissolve the solid, then add 50 mL of ethyl acetate solution, shake, separate the liquids, extract the aqueous phase with ethyl acetate three times, 30 mL each time, combine the organic phases, and concentrate with a rotary evaporator;
[0042] ④: Dissolve the concentrated solution in 15mL ethanol, reflux at 80°C for 5min, cool, precipitate a light yellow solid, filter, rinse with water, and dry under vacuum to obtain the target product with a purity of 97% (NMR analysis) and a yield of 93%. .
[0043] The compound obtained is a known comp...
Embodiment 2
[0046]
[0047] A kind of synthesis of 2-methyl-1-(2-pyridyl)indoline:
[0048] ①Add 1.33g 2-methylindoline and 1.41g 2-chloropyridine into a 50mL round bottom flask, the ratio of the substances is 1:1.25;
[0049] ②Stir directly in the air, mix evenly at room temperature, then heat to 90°C, the system solidifies after 24 minutes, stops the reaction, and cools to room temperature;
[0050] ③Add 50mL saturated NaHCO to the system 3 solution, dissolve the solid, then add 50 mL of ethyl acetate solution, shake, separate the liquids, extract the aqueous phase with ethyl acetate three times, 30 mL each time, combine the organic phases, and concentrate with a rotary evaporator;
[0051] ④: Dissolve the concentrated solution in 15mL ethanol, reflux at 80°C for 5min, cool, precipitate a light yellow solid, filter, rinse with water, and dry under vacuum to obtain the target product with a purity of 96% (NMR analysis) and a yield of 81%. .
[0052] The compound obtained is a known...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com