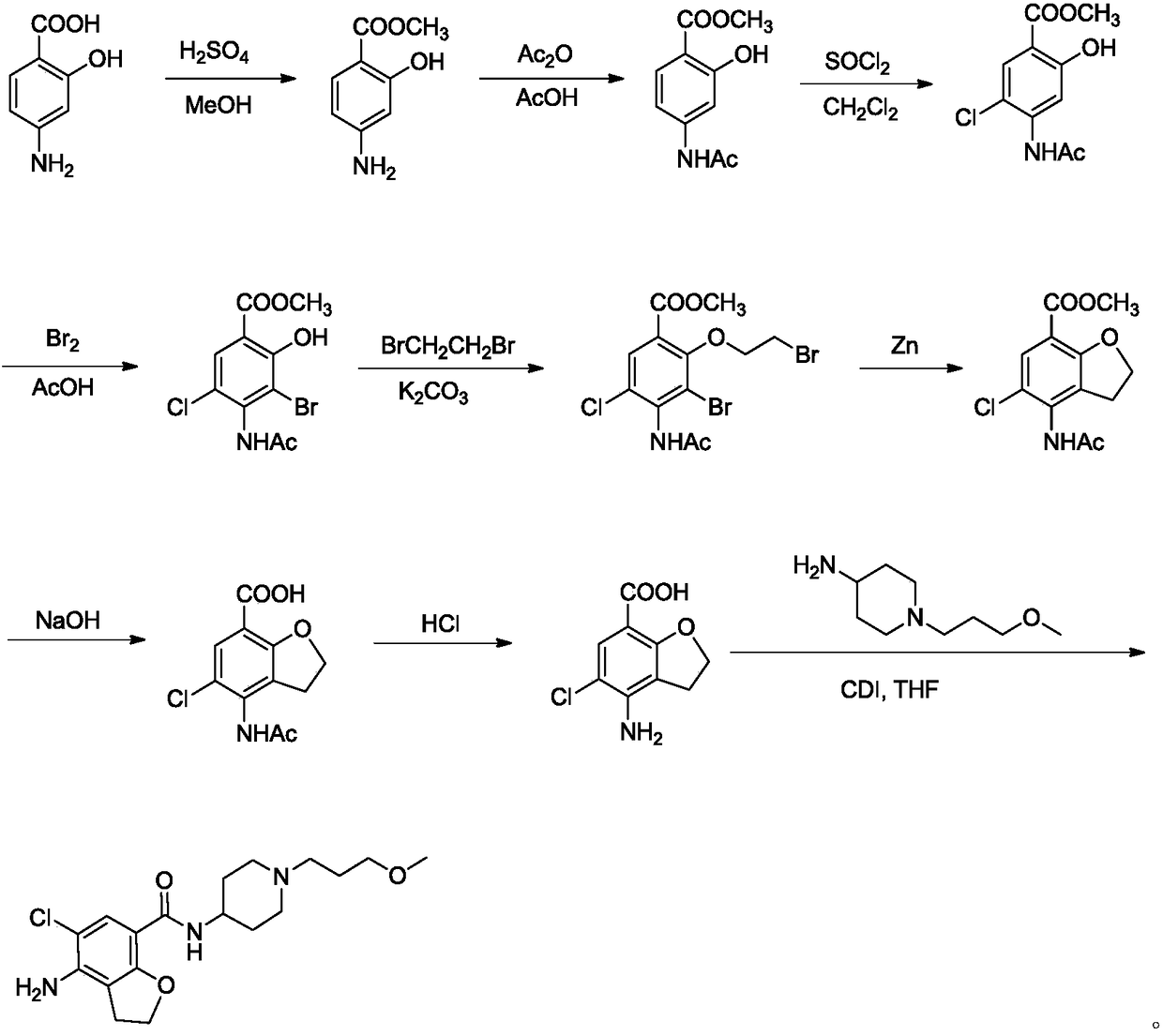
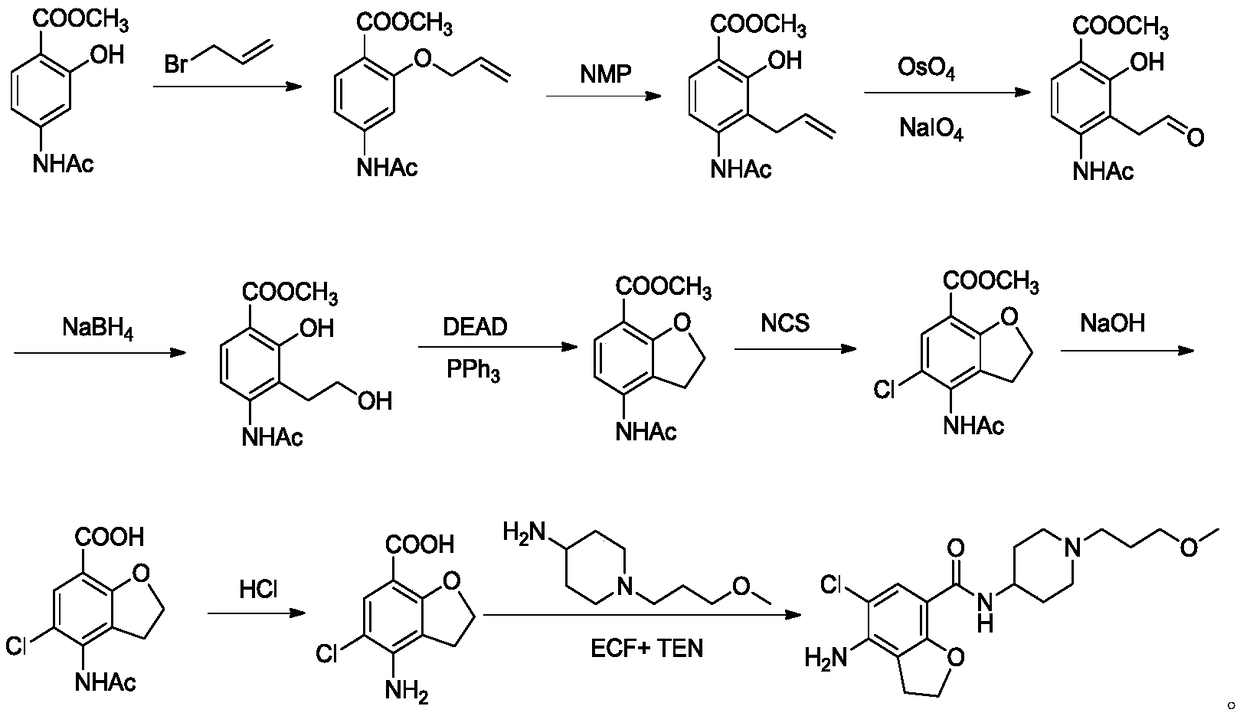
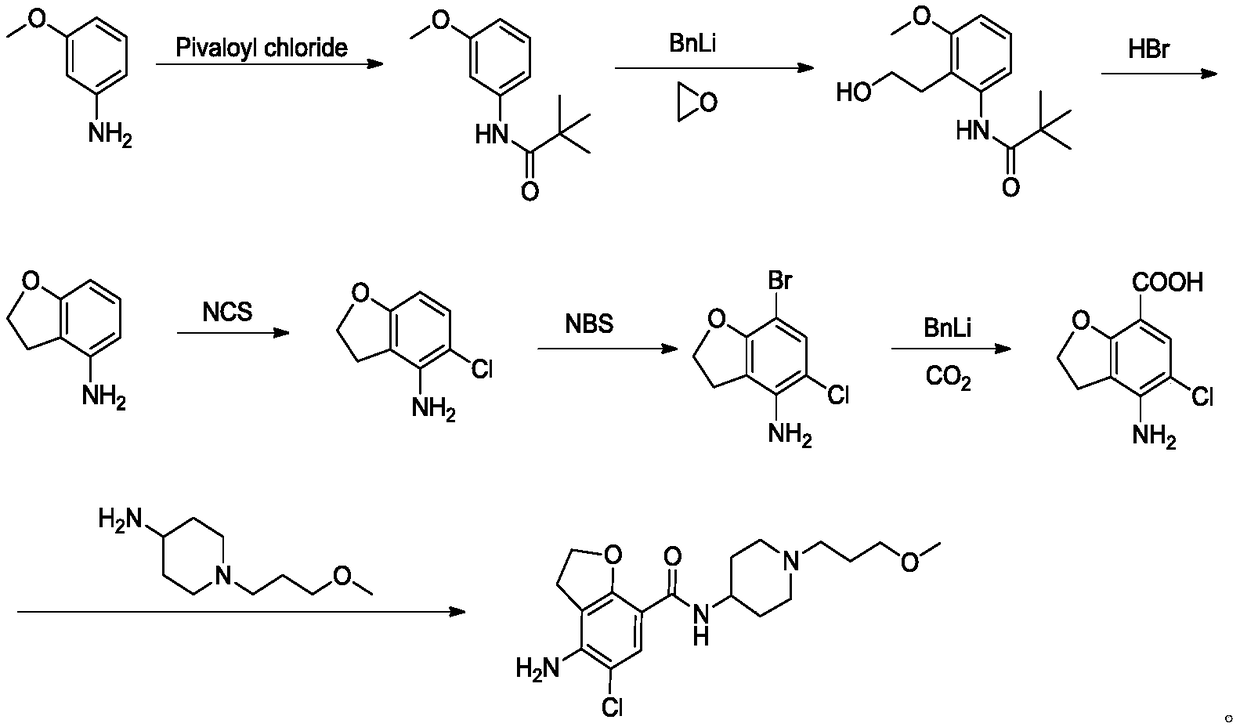
Method for preparing prucalopride
A technology of methylacetanilide and reaction, which is applied in the field of preparation of prucalopride, can solve problems such as inconvenient operation, and achieve the effects of less by-products, improved purity and yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Step 1, the preparation of 3-chloro-4-methylacetanilide: Take 141g of 3-chloro-4-methylaniline, dissolve it in 200mL ethyl acetate, add 102g of acetic anhydride dropwise under ice bath, after the dropwise addition, The temperature was raised to room temperature, and the reaction was continued for 12 hours. After the reaction, the reaction solution was washed with water until neutral, dried and filtered, and the filtrate was distilled under reduced pressure to obtain 175 g of a white solid.
[0048] Step 2, the preparation of 3-(2-hydroxyethoxy)-4-methylacetanilide: take 183g of 3-chloro-4-methylacetanilide, dissolve it in 400mL dimethyl carbonate, add ethylene glycol 62g , potassium hydroxide 100g, heated to reflux for 12h, after the reaction, filtered to remove the solid, the filtrate was washed with water, the organic layer was dried and filtered, the filtrate was decompressed to recover the solvent, and the residual solid petroleum ether:ethyl acetate=2:1 was recrysta...
Embodiment 2
[0057] Step 1, the preparation of 3-chloro-4-methylacetanilide: take 141g of 3-chloro-4-methylaniline, dissolve it in 200mL ethyl acetate, add 110g of acetic anhydride dropwise under ice bath, after the dropwise addition, The temperature was raised to room temperature, and the reaction was continued for 12 hours. After the reaction, the reaction solution was washed with water until neutral, dried and filtered, and the filtrate was distilled under reduced pressure to obtain 181 g of a white solid.
[0058] Step 2, the preparation of 3-(2-hydroxyethoxy)-4-methylacetanilide: take 183g of 3-chloro-4-methylacetanilide, dissolve it in 400mL dimethyl carbonate, add ethylene glycol 70g , potassium hydroxide 100g, heated to reflux for 12h, after the reaction, filtered to remove the solid, the filtrate was washed with water, the organic layer was dried and filtered, the filtrate was decompressed to recover the solvent, and the residual solid petroleum ether:ethyl acetate=2:1 was recrysta...
Embodiment 3
[0067] Step 1, the preparation of 3-chloro-4-methylacetanilide: Take 141g of 3-chloro-4-methylaniline, dissolve it in 200mL ethyl acetate, add 120g of acetic anhydride dropwise under ice bath, after the dropwise addition, The temperature was raised to room temperature, and the reaction was continued for 12 hours. After the reaction, the reaction solution was washed with water until neutral, dried and filtered, and the filtrate was distilled under reduced pressure to obtain 185 g of a white solid.
[0068] Step 2, the preparation of 3-(2-hydroxyethoxy)-4-methylacetanilide: Take 183g of 3-chloro-4-methylacetanilide, dissolve it in 400mL dimethyl carbonate, add ethylene glycol 76g , potassium hydroxide 100g, heated to reflux for 12h, after the reaction, filtered to remove the solid, the filtrate was washed with water, the organic layer was dried and filtered, the filtrate was decompressed to recover the solvent, and the residual solid petroleum ether:ethyl acetate=2:1 was recrysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com