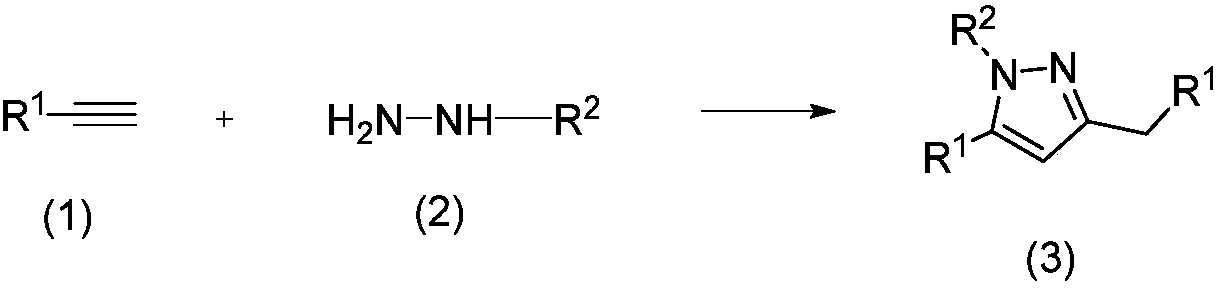
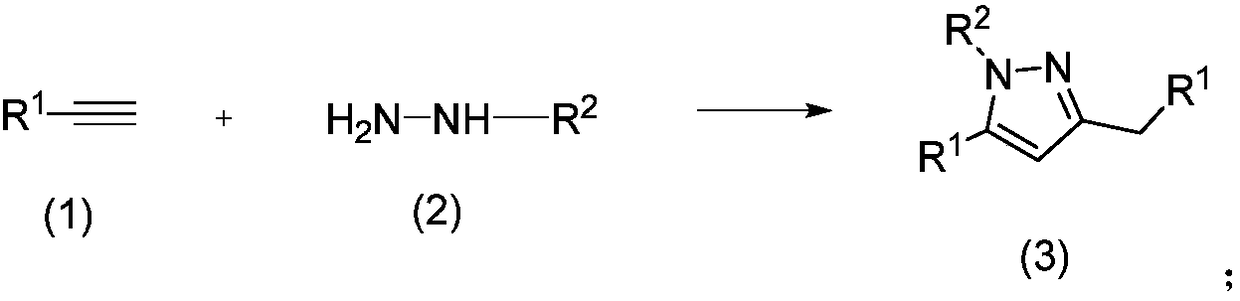
Method for preparing polysubstituted pyrazole through one-pot reaction of substituted alkyne and hydrazine or hydrazine substitute
A technology of substitution and multi-substitution, applied in the field of preparation of pharmaceutical intermediates, can solve problems such as unreported reaction of substituted pyrazole compounds, and achieve the effects of cheap raw materials, simple process and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 2ml of solvent dimethyl sulfoxide, 0.102g (1mmol) of phenylacetylene, and 0.038g (0.2mmol) of cuprous iodide to a 10ml reaction tube in turn, irradiate with 12W blue LED for 7 hours, and react at room temperature. After about 8 hours of reaction, phenylacetylene disappeared completely, then 0.2 g (4 mmol) of hydrazine hydrate and 12.8 mg (0.02 mmol) of photocatalyst methylene blue were added, and the reaction at room temperature was continued for 48 hours. After the reaction, it was extracted three times with water and ethyl acetate. The aqueous layer was removed, and the organic layer was dried over anhydrous sodium sulfate. The solvent was removed by a rotary evaporator, and then purified by silica gel chromatography (ethyl acetate / petroleum ether=1 / 10) to obtain pure 3-benzyl-5-phenyl-1H-pyrazole in a yield of 77%. 1 H NMR (400MHz, CDCl 3 )δ9.93(brs,1H),7.74-7.71(d,J=8.0Hz,2H),7.38-7.24(m,8H),6.37(s,1H),3.99(s,3H). 13 CNMR (100MHz, CDCl 3 )δ149.0, 147.6, 138.7...
Embodiment 2
[0019] In Reaction Example 1, except that 0.102 g (1 mmol) of phenylacetylene was changed to 0.116 g (1 mmol) of 1-ethynyl-4-methylbenzene, the reaction was carried out in the same manner as in Example 1. The yield of 3-(4-methylbenzyl)-5-(4-methylphenyl)-1H-pyrazole was 71%. H NMR (400MHz, CDCl 3 )δ9.12(brs,1H),7.47-7.45(d,J=8.0Hz,2H),7.08-7.04(m,6H),6.31(s,1H),3.87(s,2H),2.27(s ,3H),2.24(s,3H). 13 C NMR (100MHz, CDCl 3 )δ149.0, 147.8, 137.8, 136.2, 135.4, 129.4, 129.3, 129.1, 128.6, 125.5, 101.7, 32.8, 21.3, 21.0. HRMS (ESI) calcd for C 18 h 19 N 2 (M+H) + 263.1556, found 263.1548.
Embodiment 3
[0021] In Reaction Example 1, except that 0.102 grams (1 mmol) of phenylacetylene was changed to 0.116 grams (1 moml) of 1-ethynyl-3-methylbenzene, the reaction was carried out in the same manner as in Example 1. The yield of 3-(3-methylbenzyl)-5-(3-methylphenyl)-1H-pyrazole was 64%. 1 H NMR (400MHz, CDCl 3 )δ10.75(brs,1H),7.47-7.43(m,2H),7.83-7.11(m,2H),7.05-6.96(m,4H),6.26(s,1H),3.86(s,2H) ,2.26(s,3H),2.25(s,3H). 13 C NMR (100MHz, CDCl 3 )δ149.1,147.8,138.7,138.3,138.2,132.0,129.6,128.7,128.6,128.5,127.3,126.5,125.8,122.9,102.0,33.1,21.4,21.3.HRMS(ESI)calcd for C 18 h 19 N 2 (M+H) + 263.1458, found 263.1464.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com