Targeting nano carrier bearing nucleoside anti-tumor drugs and preparation method and application thereof
An anti-tumor drug and nanocarrier technology, applied in the field of medicine, can solve the problems of serious side reactions, poor biocompatibility and degradability, and unsatisfactory drug effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Synthesis of nucleoside antineoplastic drug phosphoramidite intermediates
[0081] In order to integrate nucleoside antineoplastic drugs into DNA single strands, nucleoside antineoplastic drugs must first be converted into corresponding phosphoramidite intermediates through chemical synthesis. Take deoxyfluridine as an example below, and carry out the synthesis of phosphoramidite intermediates according to the following synthetic route.
[0082] First, weigh 4,4-dimethoxytrityl chloride (DMT-Cl) (1.860g, 5.5mmol) and deoxyfluridine (1.231g, 5mmol), add 50mL of anhydrous pyridine, stir well, The reaction was stirred at room temperature for 4h. Then add 5mL of methanol, continue to stir the reaction for 30min. The solvent was removed by rotary evaporation under reduced pressure, and the product was separated by column chromatography to obtain a white powder (compound 2) with a yield of 80%. 1H NMR (400MHz, DMSO-d6) δ (ppm): 11.86 (1H, br s, NH), 7.88 (1H, d, ...
Embodiment 2
[0089] Example 2 Synthesis of DNA single strands supporting oligonucleoside antineoplastic drugs
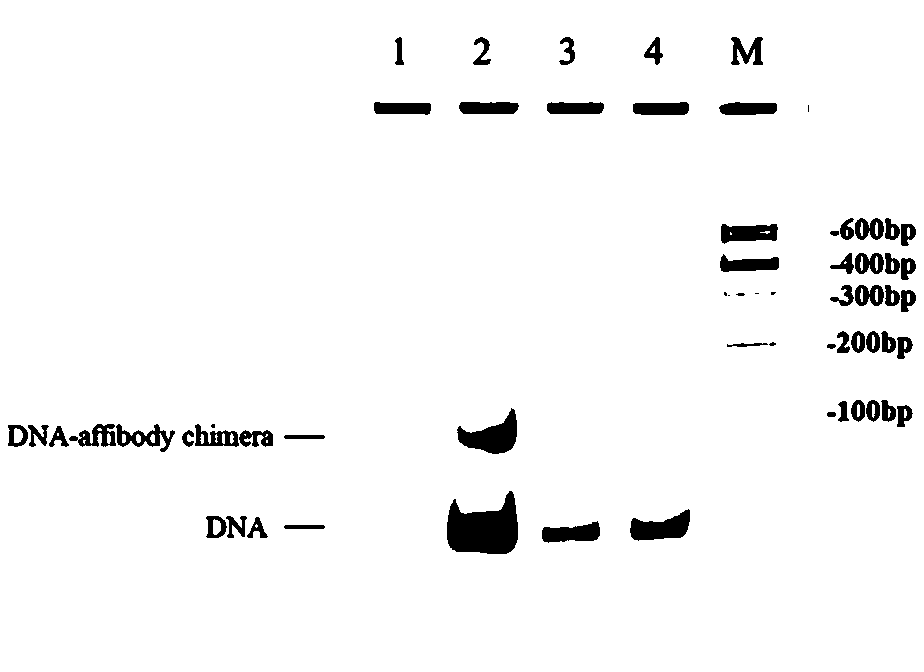
[0090] The oligodeoxyfluorouridine (n=10) sequence was connected to the 5' ends of the four DNA single strands of the DNA tetrahedron through DNA solid-phase synthesis technology (F represents deoxyfluridine), and one of the DNA single strands 3' modified NH2. The nucleotide sequence of each DNA single strand is as follows:
[0091] A13F-NH2:
[0092] 5’-FFFFFFFFFFACACTACGTCAGAACAGCTTGCATCACTGGTCACCAGAGTA-NH 2 -3';
[0093] B13F: 5'-FFFFFFFFFFACGAGCGAGTTGATGTGATGCAAGCTGAATGCGAGGGTCCT-3';
[0094]C13F:5'-FFFFFFFFFFCAACTCGCTCGTAACTACACTGTGCAATACTCTGGTGACC-3';
[0095] D13F: 5'-FFFFFFFFFFCTGACGTAGTGTATGCACAGTGTAGTAAGGACCCTCGCAT-3'.
[0096] Freeze-dried powders of four DNA single strands were obtained through DNA synthesis and stored at -20°C for later use.
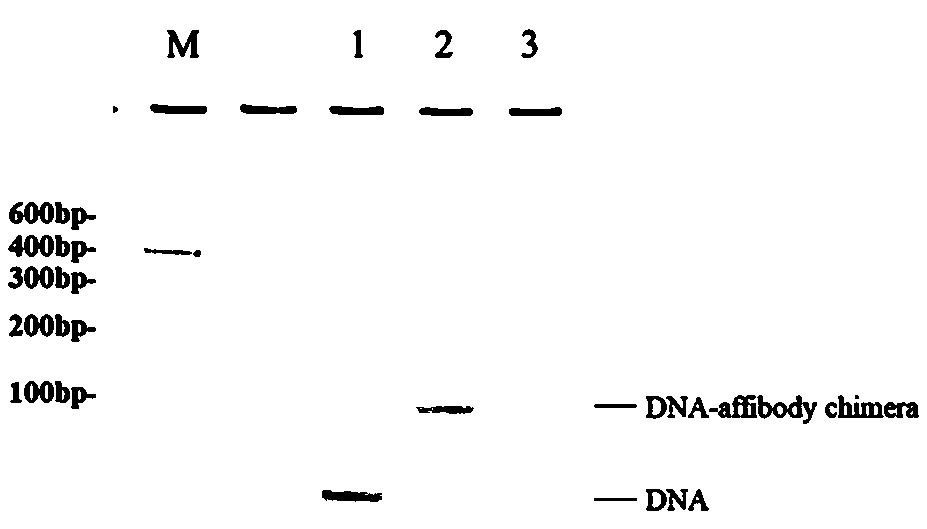
[0097] The oligomeric gemcitabine (n=16) sequence was connected to the 5' end of the four DNA single strands of the DN...
Embodiment 3
[0107] Example 3 Fermentative production and purification of affibody molecules
[0108] The sequence of the affibody molecule used in this example is:
[0109] MIHHHHHHLQVDNKFNKEMRNAYWEIALLPNLNNQQKRAFIRSLYDDPSQSANLLAEAKKLNDAQAPKVDC.
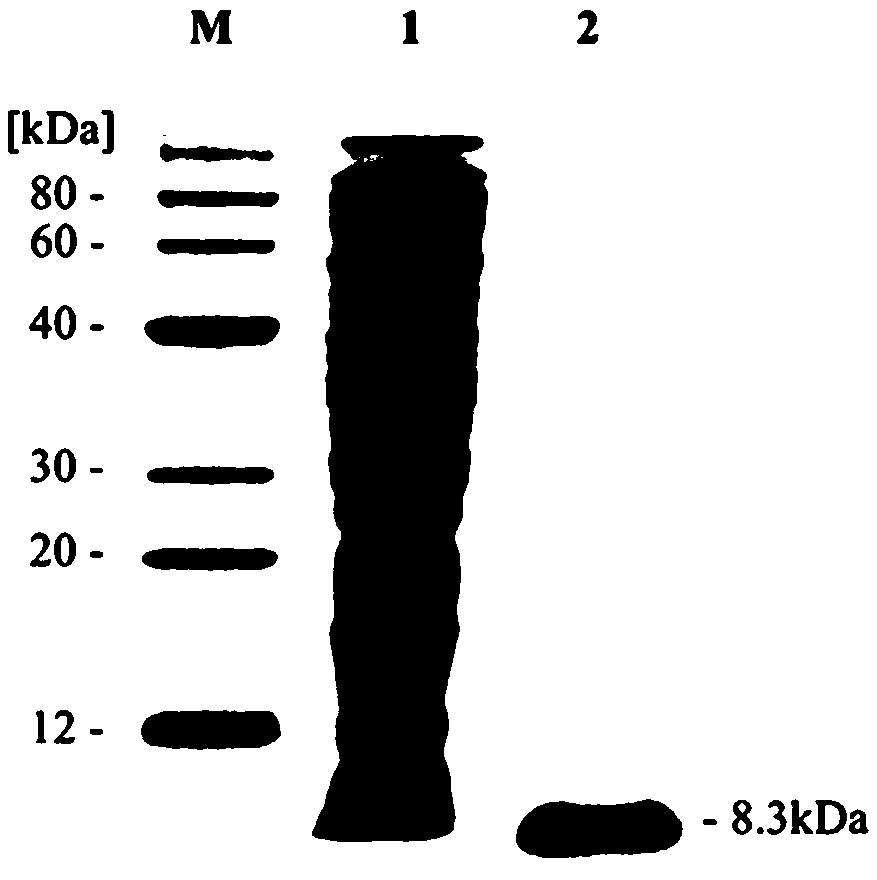
[0110] Affibody was expressed and produced in Escherichia coli BL21(DE3) cells. The seed liquid culture conditions are: 37° C., 200 rpm shaking, 80 mL of LB medium (100 μg / mL ampicillin) in the culture liquid for 10 hours. The culture condition of the 5L fermenter is: 10% (v / v) inoculum amount, inoculated into a 5L fermenter filled with 2.0L LB medium (100 μg / mL ampicillin). At the same time, 0.5 mM final concentration of IPTG was added to the medium and the temperature was lowered to 30 °C. During the entire fermentation process, the pH of the culture solution was maintained at 7.0 by automatically adding ammonia solution. After the fermentation was completed, Escherichia coli containing affibody was obtained by centrifugation, and the Esch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com