Lithium zirconium phosphate fast ionic conductor coated lithium nickel cobalt aluminate positive electrode material and preparation method thereof
A technology of nickel cobalt lithium aluminate and lithium zirconium phosphate, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problem of difficulty in film formation or uniform mixing, limited improvement in ternary cycle performance, and impact on ternary cycle. performance and other issues, to achieve good high-rate discharge performance, good cycle stability, and the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
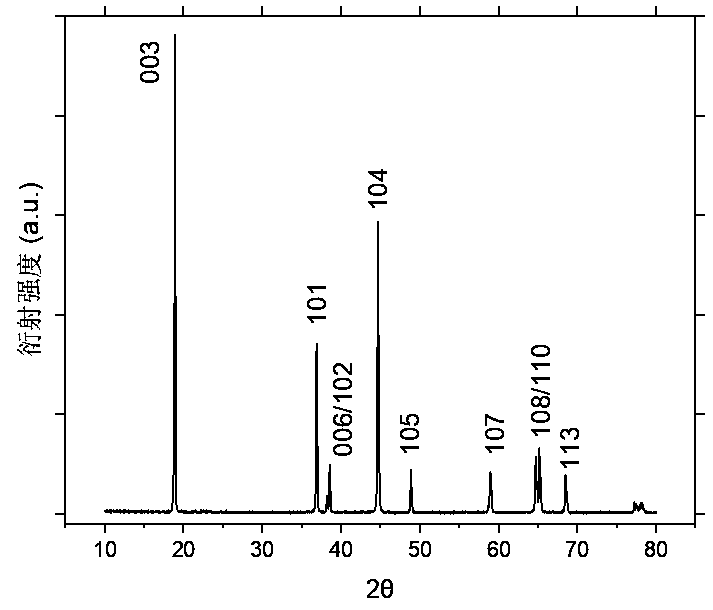
Image
Examples
Embodiment 1
[0038] (1) Weigh 0.90513g (0.002108287mol) zirconium nitrate pentahydrate (relative molecular mass 429.32), add water to dissolve, transfer to a 50ml volumetric flask, add deionized water to volume, weigh 0.32867g (0.003162431mol) dihydrogen phosphate Lithium (relative molecular mass: 103.93), dissolved in water, transferred to a 50ml volumetric flask, added water to volume. The ratio of zirconium and phosphorus is 2:3, (under the condition of this ratio, 1ml of zirconium nitrate and 1ml of lithium dihydrogen phosphate are mixed to obtain the required ratio of 1wt% lithium zirconium phosphate coated on the ternary material, that is, two Mix 1ml of each solution, which is the required amount for 1wt% coating. 0.5ml of each of the two solutions is the required amount for 0.5wt% coating, and the rest of the coating amount can be deduced in the same way. The volume of the solution can be used as the proportioning amount, and the operation is simplified, the same below, and will no...
Embodiment 2
[0042] (1) Weigh 0.9051g (0.002108mol) zirconium nitrate pentahydrate, add water to dissolve and transfer to a 50ml volumetric flask, add deionized water to volume, weigh 0.3287g (0.00316mol) lithium dihydrogen phosphate, add water to dissolve and transfer To a 50ml volumetric flask, add water to volume. The molar ratio of phosphorus to zirconium is 2:3.
[0043] (2) Use a pipette to take 0.5ml of the zirconium nitrate solution described in (1) above, add 75ml of alcohol, stir well, then add 0.5ml of the lithium dihydrogen phosphate solution described in (1) above, and stir slowly.
[0044] (3) Take 0.5g lithium nickel cobalt aluminate and add it to the above solution (2), heat and stir at 90°C for 1.5h until evaporated to dryness, then directly transfer to a tube furnace, under an oxygen atmosphere, heat at 5°C / min heating rate, heated to 650 degrees and kept for 1 hour, to obtain nickel cobalt lithium aluminate cathode material coated with lithium zirconium phosphate fast ...
Embodiment 3
[0046] (1) Weigh 0.9051g (0.002108mol) zirconium nitrate pentahydrate, add water to dissolve and transfer to a 50ml volumetric flask, add deionized water to volume, weigh 0.3287g (0.00316mol) lithium dihydrogen phosphate, add water to dissolve and transfer To a 50ml volumetric flask, add water to volume. The molar ratio of phosphorus to zirconium is 2:3.
[0047] (2) Use a pipette to take 0.5ml of the zirconium nitrate solution described in (1) above, add it to 75ml of alcohol, stir evenly, then add 0.5ml of the lithium dihydrogen phosphate solution described in (1) above, and stir slowly.
[0048] (3) Add 0.5g lithium nickel-cobalt aluminate to the solution of (2) above, heat, stir and evaporate to dryness at 90°C for 1.5h, and when the mixed slurry becomes viscous, about 3-5ml, stop stirring, Slowly evaporate the solution to dryness, and then transfer it to an oven for drying. After drying, transfer it to a tube furnace, heat it to 650 degrees for 1 hour at a heating rate o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com