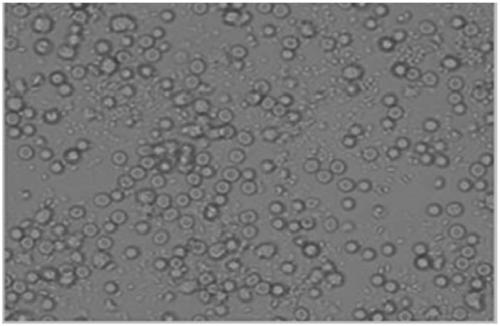
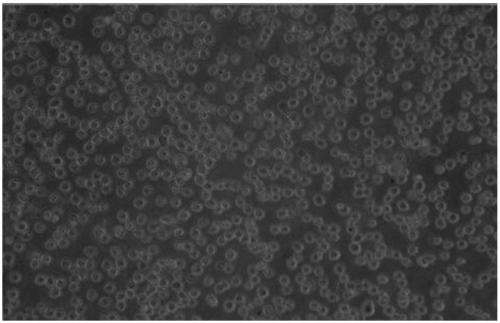
Culture medium capable of amplifying number of endothelial progenitor cells
A technology of endothelial progenitor cells and culture medium, which is applied in the field of culture medium for expanding the number of endothelial progenitor cells, which can solve the problems of impaired function and low number of endothelial progenitor cells, reduce pollution, facilitate separation, and have good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In this example, under the condition that EGM-2 is used as the basal medium, 10% fetal bovine serum, 5 g / L soybean plant hydrolyzed protein, 10 μg / L VEGF, 2 μg / L bFGF, and 20 μg / L epidermal growth factor are used , 50 μM Vitamin C, 1 μM AR-A014418.
[0016] This embodiment reduces the amount of fetal bovine serum used in conventional medium, uses soybean plant hydrolyzed protein, and its cost is lower than conventional medium, and solves the problem of premature cell death and unsaturated morphology caused by reducing the use of fetal bovine serum question, the number of endothelial progenitor cells was expanded using AR-A014418.
Embodiment 2
[0018] In this example, under the condition that EGM-2 is used as the basal medium, 10% fetal bovine serum is used, and 10 g / L soybean plant hydrolyzed protein, 50 μg / L VEGF, 10 μg / L bFGF, and 100 μg are added to the medium. / L of epidermal growth factor, 200 μM of vitamin C, 5 μM of AR-A014418.
[0019] This embodiment reduces the amount of fetal bovine serum used in conventional medium, uses soybean plant hydrolyzed protein, and its cost is lower than conventional medium, and solves the problem of premature cell death and unsaturated morphology caused by reducing the use of fetal bovine serum Question, using AR-A014418 to expand endothelial progenitor cell numbers.
Embodiment 3
[0021] In this example, under the condition that EGM-2 is used as the basal medium, 10% fetal bovine serum is used, and 7 g / L soybean plant hydrolyzed protein, 30 μg / L VEGF, 7 μg / L bFGF, 70 μg / L of epidermal growth factor, 100 μM of vitamin C, 3 μM of AR-A014418.
[0022] This embodiment reduces the amount of fetal bovine serum used in conventional medium, uses soybean plant hydrolyzed protein, and its cost is lower than conventional medium, and solves the problem of premature cell death and unsaturated morphology caused by reducing the use of fetal bovine serum Question, using AR-A014418 to expand endothelial progenitor cell numbers.
[0023] The low-serum culture medium developed by the present invention is similar in shape to conventionally cultured 20% fetal bovine serum, reduces pollution, is beneficial to the separation of downstream products of cells, and has good safety; compared with commercial culture medium, the cost is reduced, Suitable for bulk production of end...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com