Synthesis method and anti-tumor application of bisindole-pyridine derivatives
A technology of indolopyridines and derivatives is applied in the directions of antitumor drugs, drug combinations, organic chemistry, etc., and achieves the effects of simple operation process, low cost and strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
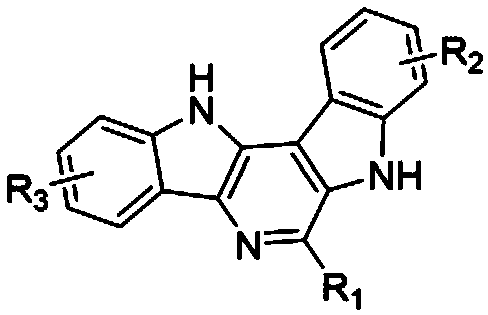
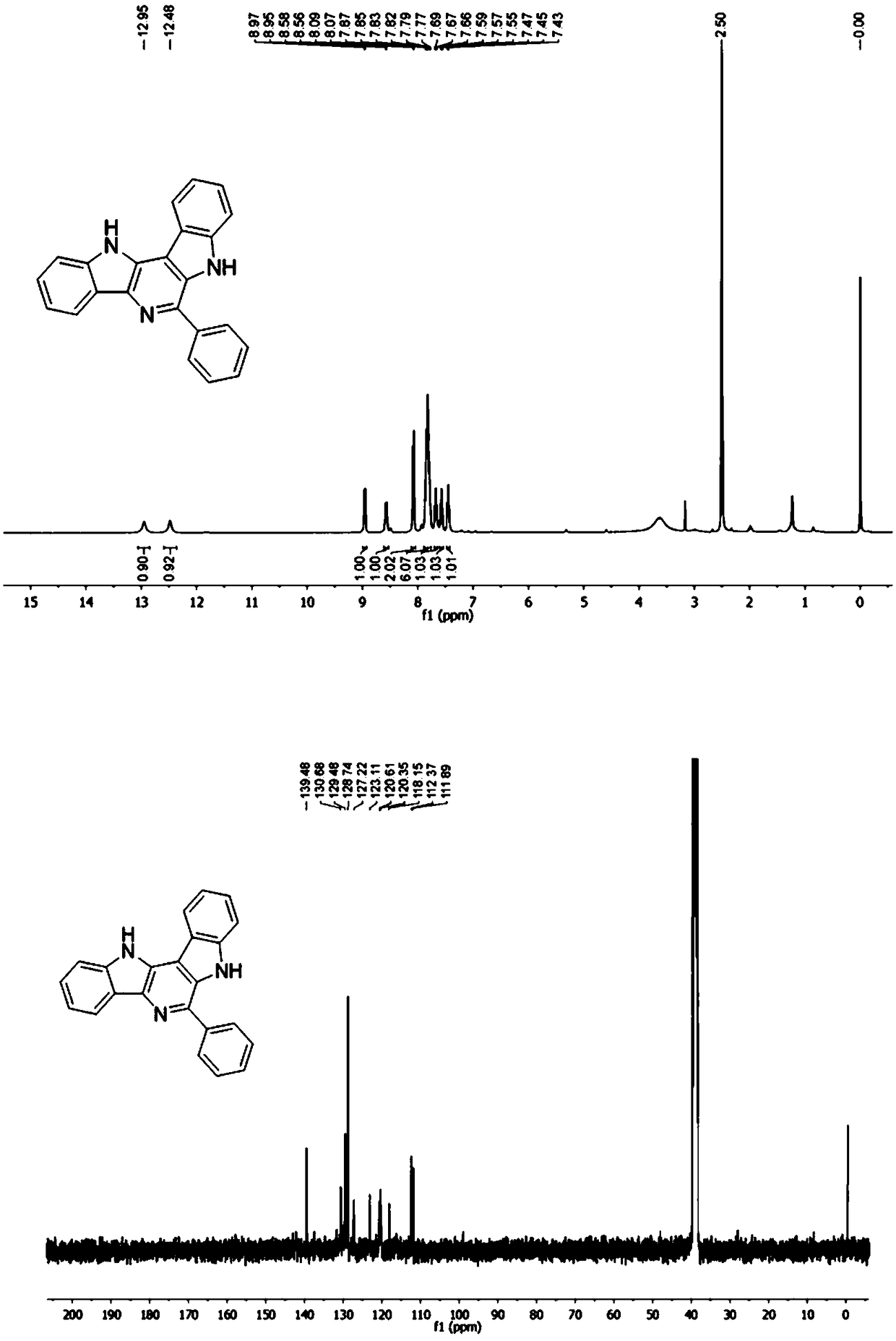
[0051] where R 1 is phenyl, R 2 is a hydrogen atom, R3 is a hydrogen atom, that is, the synthesis of 6-phenyl-7,12-dihydropyrido[3,2-b:5,4-b']diindole, the specific steps are as follows:
[0052] In a 5 mL microwave reaction tube, dissolve 0.5 mmol of benzaldehyde and 0.5 mmol of Boc-protected indoleamine in 2 mL of methanol, stir at room temperature for 5 minutes, then add 0.05 mmol of perchloric acid and 0.5 mmol of benzyl isocyanide, respectively . and placed in a microwave reactor for 100 o C for 10 minutes. After the completion of the reaction, cool to room temperature, pour the reaction solution into 15 mL ethyl acetate, wash with saturated sodium bicarbonate and brine, dry the organic phase with anhydrous sodium sulfate, concentrate, and use ethyl acetate / n-hexane (20-80%) gradient The target compound 6-phenyl-7,12-dihydropyrido[3,2-b:5,4-b']diindole was obtained by elution and separation with a yield of 76%.
[0053] 1 H NMR (400 MHz, DMSO- d 6 ) δ 12.95 (s, 1H...
Embodiment 2
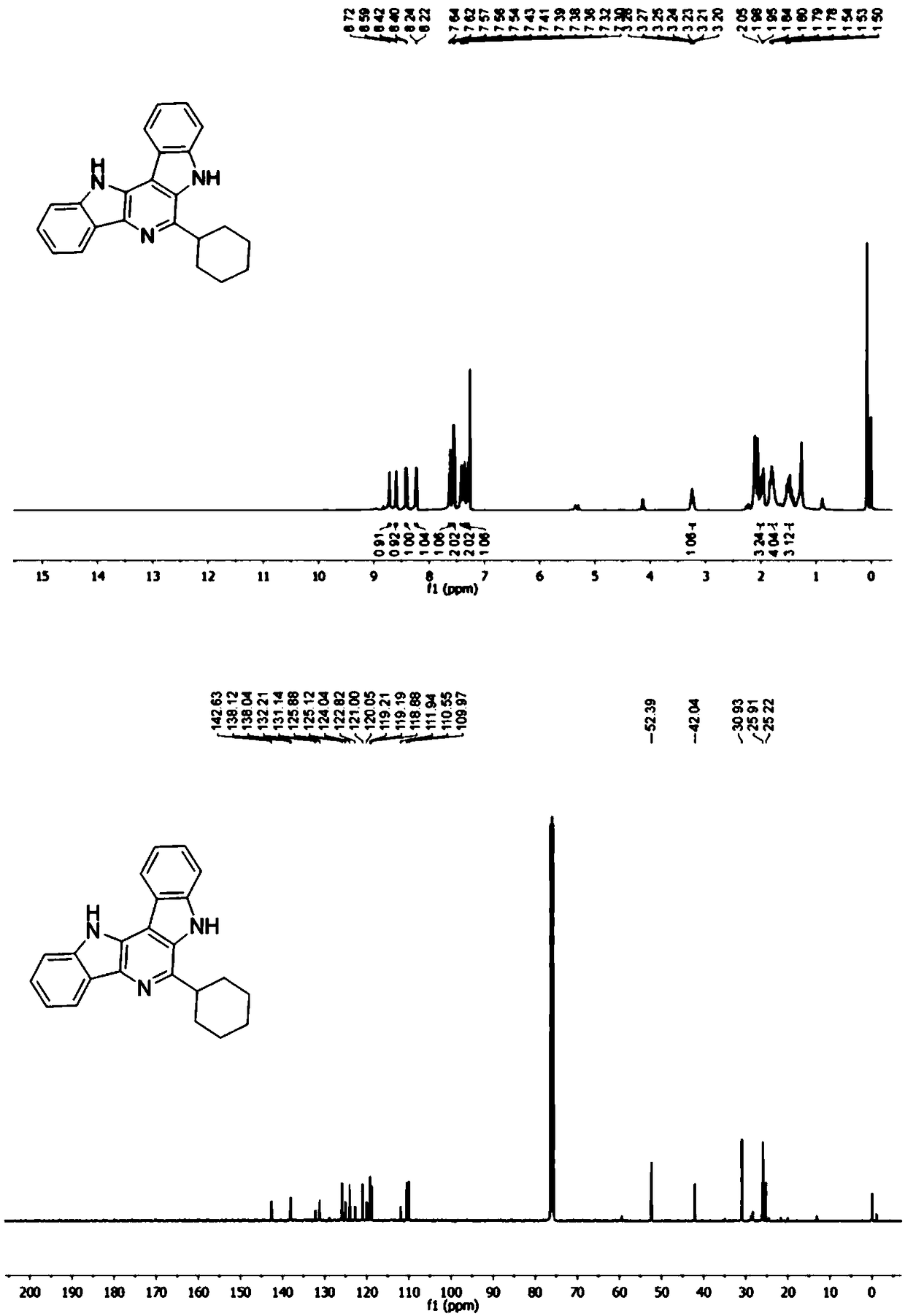
[0055] where R 1 is an alkyl group, R 2 is a hydrogen atom, R 3 is a hydrogen atom, that is, the synthesis of 6-cyclohexyl-7,12-dihydropyrido[3,2-b:5,4-b']diindole, the specific steps are as follows:
[0056] In a 5 mL microwave reaction tube, dissolve 0.5 mmol of cyclohexylaldehyde and 0.5 mmol of Boc-protected indoleamine in 2 mL of methanol, stir at room temperature for 5 minutes, and then add 0.05 mmol of perchloric acid and 0.5 mmol of benzyl isonitrile . and placed in a microwave reactor for 100 o C for 10 minutes. After the completion of the reaction, cool to room temperature, pour the reaction solution into 15 mL ethyl acetate, wash with saturated sodium bicarbonate and brine, dry the organic phase with anhydrous sodium sulfate, concentrate, and use ethyl acetate / n-hexane (20-80%) gradient The target compound 6-cyclohexyl-7,12-dihydropyrido[3,2-b:5,4-b']diindole was obtained by elution and separation with a yield of 72%.
[0057] 1 H NMR (400 MHz, CDCl 3 ) δ 8....
Embodiment 3
[0059] where R 1 is aryl, R 2 is a hydrogen atom, R 3 is a hydrogen atom, that is, the synthesis of 6-(4-bromophenyl)-7,12-dihydropyrido[3,2-b:5,4-b']diindole, the specific steps are as follows:
[0060] In a 5 mL microwave reaction tube, dissolve 0.5 mmol of 4-bromophenylaldehyde and 0.5 mmol of Boc-protected indoleamine in 2 mL of methanol, stir at room temperature for 5 minutes, and then add 0.05 mmol of perchloric acid and 0.5 mmol of benzyl isonitrile. and placed in a microwave reactor for 100 o C for 10 minutes. After the completion of the reaction, cool to room temperature, pour the reaction solution into 15 mL ethyl acetate, wash with saturated sodium bicarbonate and brine, dry the organic phase with anhydrous sodium sulfate, concentrate, and use ethyl acetate / n-hexane (20-80%) gradient The target compound 6-(4-bromophenyl)-7,12-dihydropyrido[3,2-b:5,4-b']diindole was obtained by elution and separation with a yield of 83%.
[0061] 1 H NMR (400 MHz, DMSO- d 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com