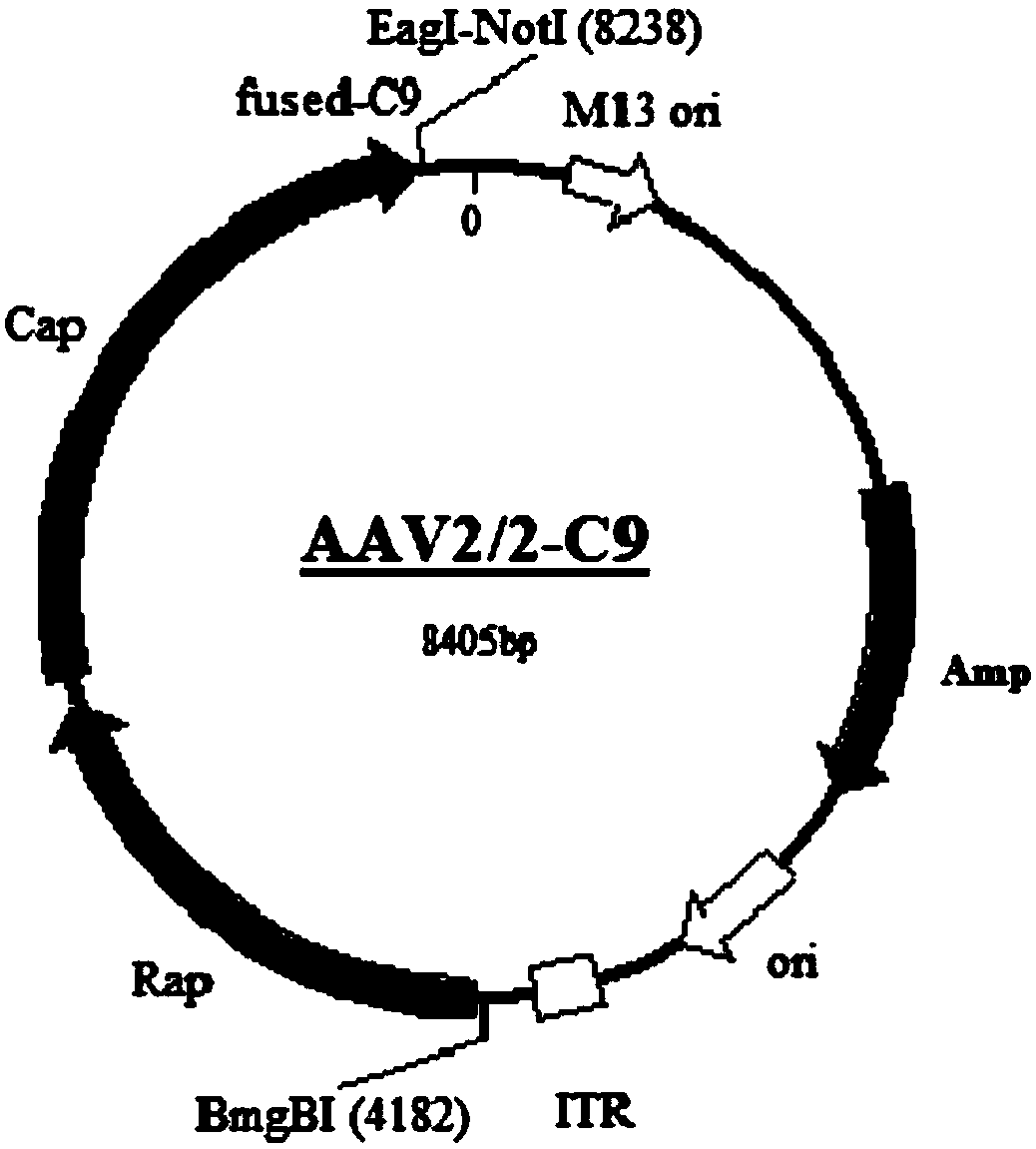
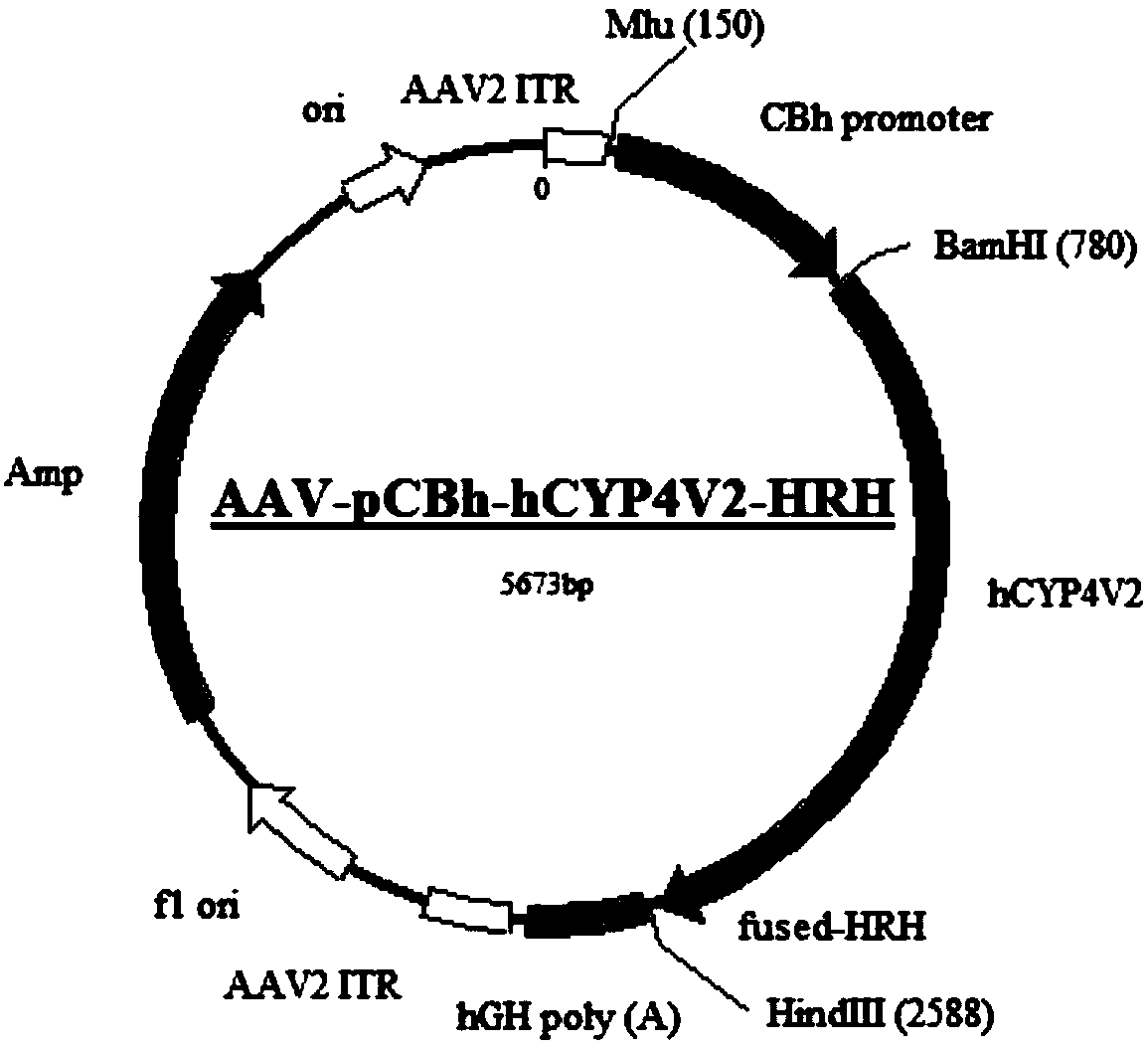
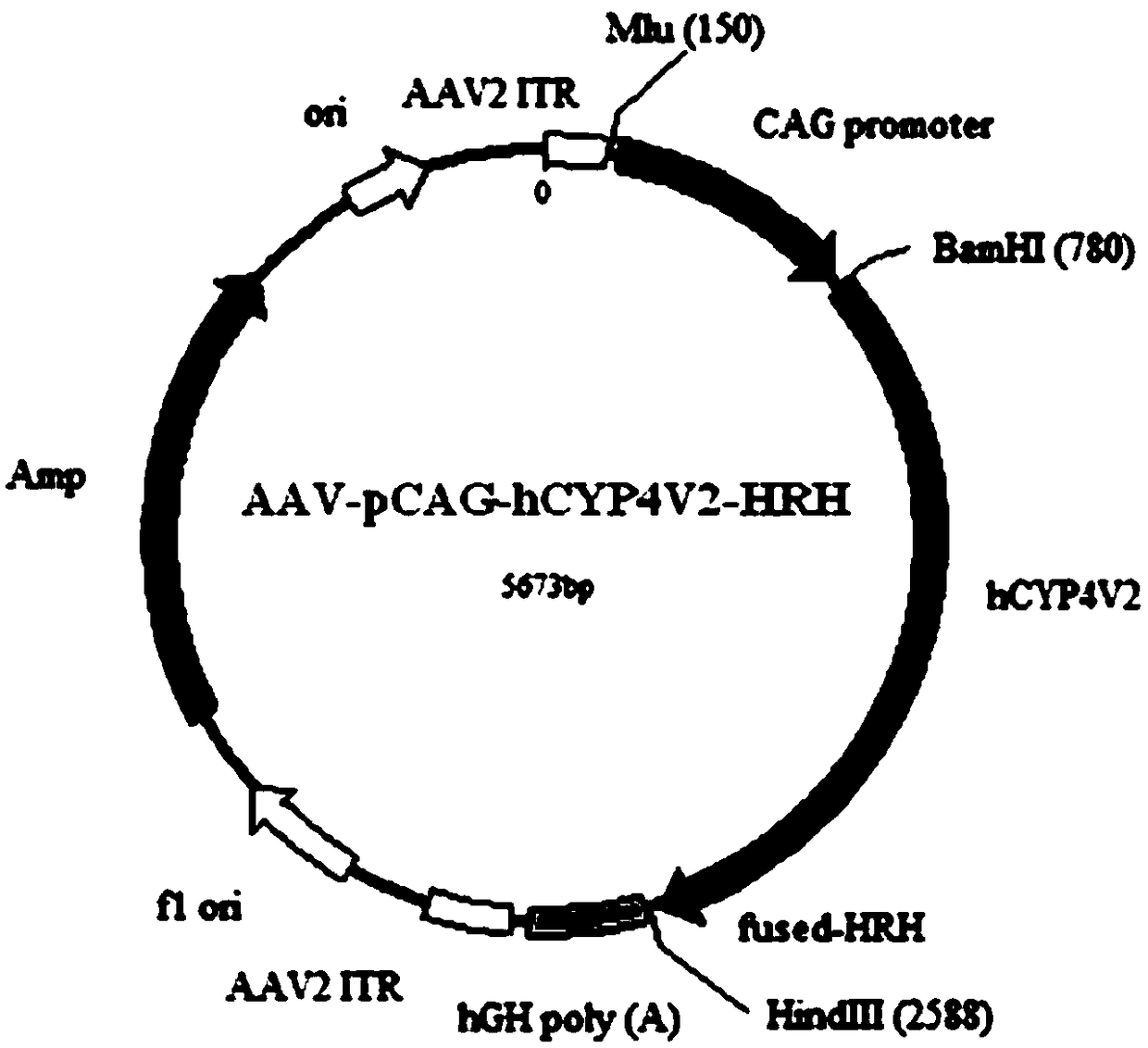
Gene carrier for treating or preventing bietti's crystalline dystrophy and application of gene carrier
A retinal pigment and gene carrier technology, which is used in the introduction of foreign genetic material, gene therapy, genetic engineering and other directions using vectors to achieve the effect of delaying retinal degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Construction and separation and purification of adenoviral vector
[0031] 1. Amplify the coding sequence of CYP4V2-HRH gene.
[0032] 1) NCBI query to obtain the cDNA sequence of human CYP4V2 gene (https: / / www.ncbi.nlm.nih.gov / nuccore / NM_207352.3), and synthesize the sequence (SEQ ID NO:1).
[0033] 2) According to the above sequence, use T2A to fuse the HRH short peptide nucleotide sequence, and send it to the company to synthesize the CYP4V2-HRH gene fragment (SEQ ID NO: 2).
[0034] 3) Design and synthesize primers, and use high-fidelity PCR to clone and amplify the CYP4V2-HRH sequence.
[0035] CYP4V2-F: CGCGGATCCATGGCGGGGCTCTGGCTGG
[0036] CYP4V2-R:GGTAAGCTTTTAGCGCGGTATGGCGC
[0037] 2. Amplify multiple general promoters
[0038] 1) Query Addgene (Cambridge non-profit plasmid resource bank) to obtain CBh, CAG, PGK and EF1α promoter sequences, plasmid ID: 42230, 16664, 21217 and 26797.
[0039] 2) Synthesize its DNA sequence (SEQ ID NO: 4 to SEQ ID...
Embodiment 2
[0084] Example 2: Verification of in vitro overexpression of adenoviral vectors
[0085] 1. Culture ARPE-19 cells in a twelve-well cell culture plate until 70% saturated.
[0086]2. Add 10000MOI AAV2-C9-pCAG-hCYP4V2-HRH and AAV2-C9-pRPE65-hCYP4V2-HRH viruses, incubate overnight, and package the corresponding AAV2-pCAG-hCYP4V2-HRH and AAV2 with unmodified AAV2 capsid plasmid - pRPE65-hCYP4V2-HRH virus was used as control.
[0087] 3. Change to fresh medium to continue culturing.
[0088] 4. After 3 days, collect the cells and extract the total protein of the cells.
[0089] 5. Western blot to verify the expression status of CYP4V2.
[0090] 6. Results such as Figure 7 Shown: 1 is the blank group without virus infection, which only expresses a very low amount of CYP4V2 protein; 2 is the AAV2-pCAG-hCYP4V2-HRH control group, the expression of CYP4V2 protein has increased, and a certain amount of HRH expression can be detected ; 3 is the AAV2-C9-pCAG-hCYP4V2-HRH group, compar...
Embodiment 3
[0091] Example 3: Functional verification of adenoviral vectors
[0092] 1. Establishment of BCD patient iPSC cell line
[0093] Using iPSC reprogramming technology (Cell.2006Aug 25; 126(4):663-76.Epub 2006Aug10.Induction of pluripotent stem cells from mouse embryonic and adultfibroblast cultures by defined factors.Takahashi K1,Yamanaka S.) to extract normal people and BCD Peripheral blood cells from the patient were cultured in CD34+ serum-free medium for 10 days, then electrotransfected with Yamanaka four-factor plasmid, added with serum-free culture for two days, then transferred to Geltrex-treated cell culture plates, and continued to grow into iPSCs in stem cell medium E8 Clones appear. Usually, iPSCs appear after two weeks. When the clone grows to be visible to the naked eye, it can be subcultured and expanded for the next step of cell differentiation experiment.
[0094] 2. BCD patient and normal human RPE cell differentiation
[0095] RPE cell differentiation was pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com