Bidirectional screening system construction method for directed evolution of lead binding protein
A technology that binds proteins and lead ions, applied in chemical instruments and methods, biochemical equipment and methods, botany equipment and methods, etc., can solve problems such as limited application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 Contains the screening plasmid chassis cell construction of two-way screening system
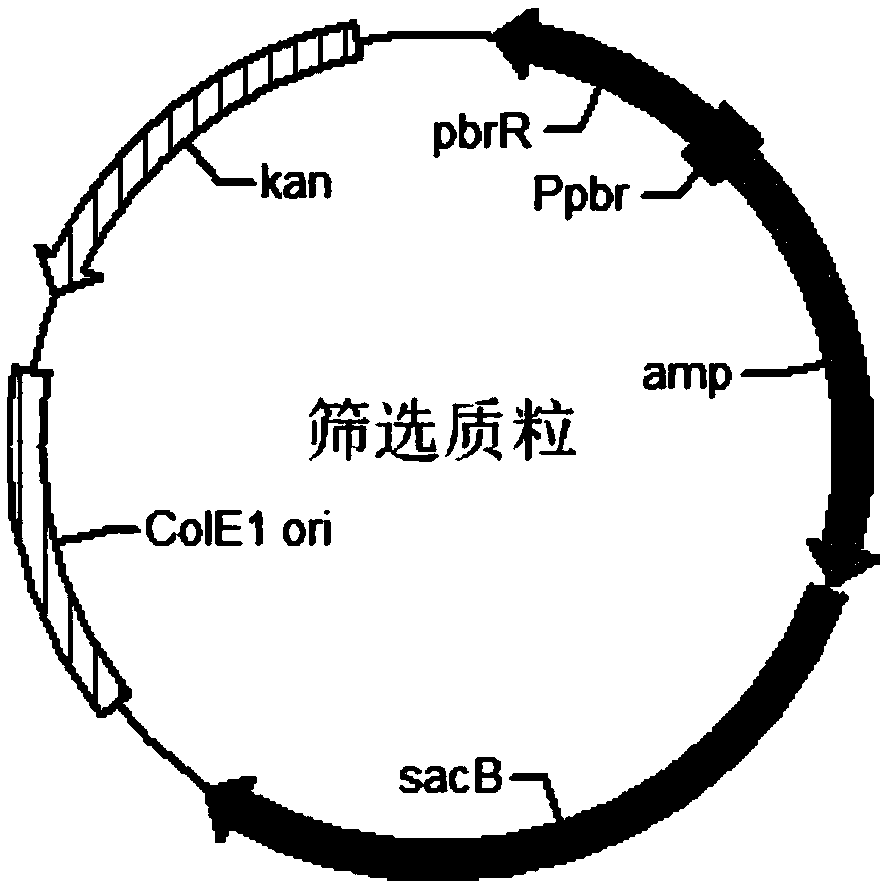
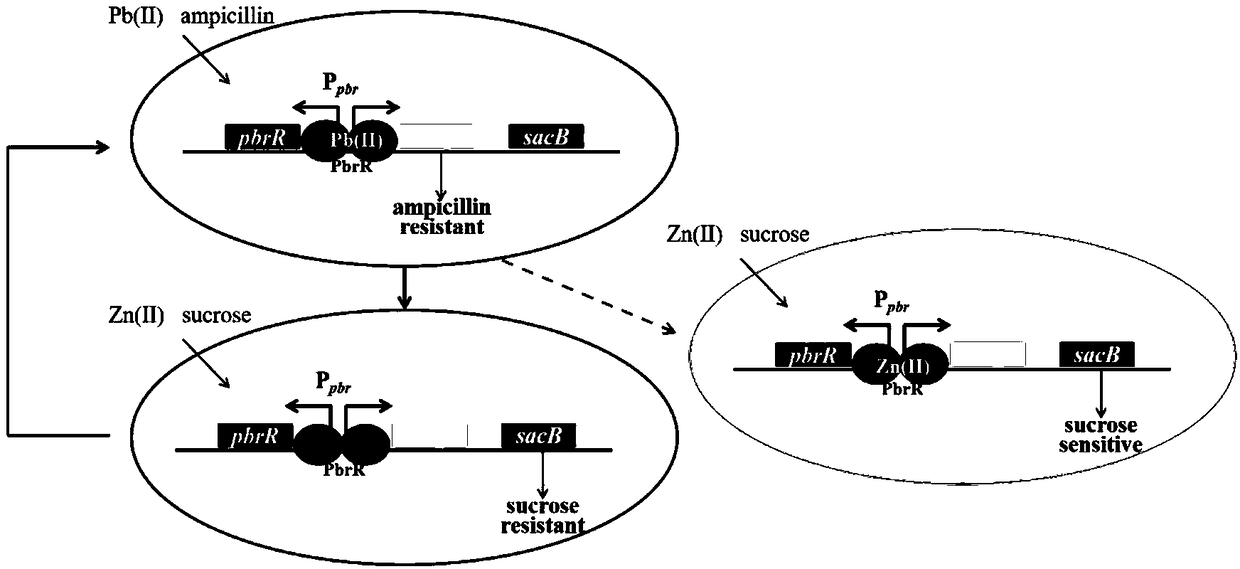
[0044] Artificial Totally Synthetic Lead Binding Protein Gene pbrR and Promoter P pbr , whose nucleotide sequence is shown in SEQ ID No.1; ampicillin resistance gene amp, whose nucleotide sequence is shown in SEQ ID No.2; fructan sucrase gene sacB, whose nucleotide sequence is shown in Shown in SEQ ID No.3. The nucleotide sequences shown in SEQ ID No.1, SEQ ID No.2, and SEQ ID No.3 and the vector backbone gene fragments were connected by Gibson Assembly, and the positive clones were screened to obtain the screened plasmid. The plasmid map is as follows: figure 1 shown. The screening mechanism of the bidirectional screening system for directed evolution of lead-binding proteins such as figure 2 shown.
[0045] Use high-fidelity DNA polymerase to configure the reaction system, and amplify gene fragments by polymerase chain reaction: vector backbone gene fragments (prim...
Embodiment 2
[0048] The expression verification of the two-way screening system under the condition of metal lead ion of embodiment 2
[0049] The screening plasmid chassis cells containing the two-way screening system were inserted into LB liquid medium with kanamycin 50 μg / mL and cultured overnight at 37°C and 220 rpm, and then transferred to 50 mL LB liquid medium until the cells grew to OD 600 About 0.6.
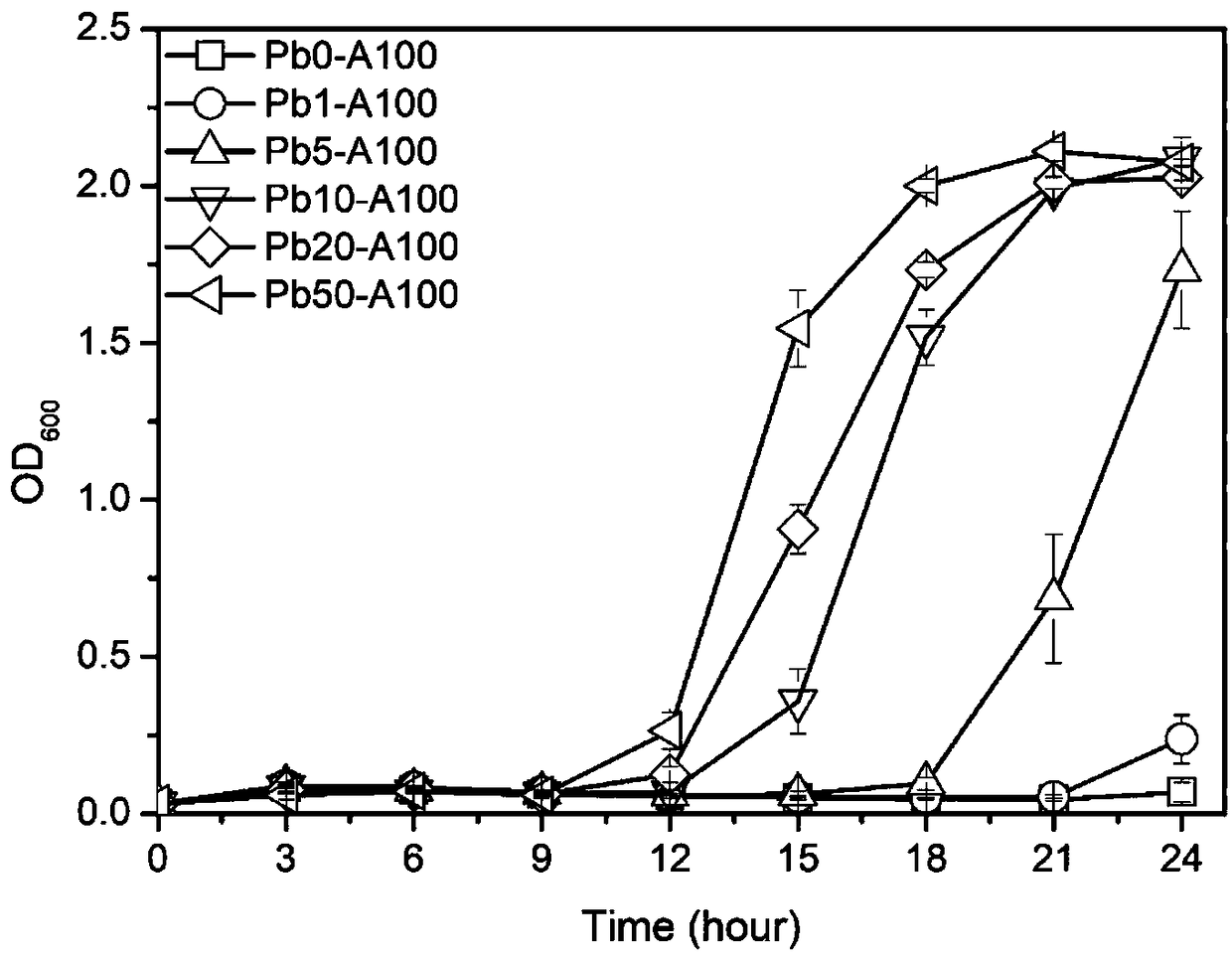
[0050] (1) Expression verification of lead ion positive screening markers: the OD 600 About 0.6 bacteria solution was transferred to 50mL LB liquid medium supplemented with lead nitrate solution and ampicillin solution at a ratio of 1%, and the final concentration of lead ions was set to a concentration gradient of 0, 1, 5, 10, 20, and 50 μM , the final concentration of ampicillin solution was 100 μg / mL, and three parallels were set for each sample. Shake flask culture was carried out at 37°C and 220rpm, and the absorbance OD at a wavelength of 600nm was measured with a UV-visible ...
Embodiment 3
[0054] The expression optimization of the two-way screening system under the condition of metal lead-zinc ion of embodiment 3
[0055] The screening plasmid chassis cells containing the two-way screening system were inserted into kanamycin-resistant LB medium and cultured overnight at 37°C and 220rpm, and then transferred to 50mL LB liquid medium until the cells grew to OD 600 About 0.6.
[0056] (1) Expression optimization of positive screening markers at the same concentration of lead and zinc ions: the OD 600 About 0.6 of the bacterial solution was transferred to 50 mL of LB liquid medium supplemented with ampicillin 100 μg / mL at a ratio of 1%, and lead nitrate solution and zinc nitrate solution were added to the final concentrations of 20 μM and 50 μM respectively. parallel. Shake flask culture was carried out at 37°C and 220rpm, and the absorbance OD at a wavelength of 600nm was measured with a UV-visible spectrophotometer every 3h 600 , draw the growth curve, such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com