A kind of room temperature phase change energy storage material and preparation method thereof
A phase change energy storage material and phase change material technology, which are applied in the field of room temperature phase change energy storage materials and their preparation, can solve the problems of phase separation, high degree of supercooling, poor thermal conductivity, etc., so as to increase stability and improve wetting. performance, effect of reducing supercooling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
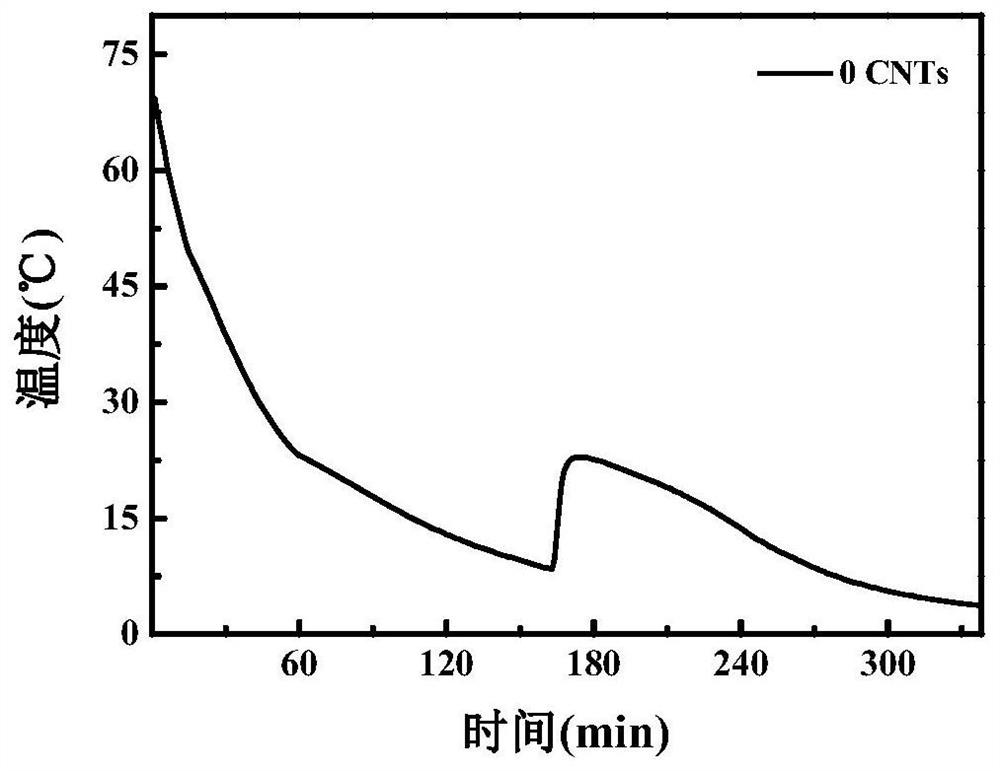
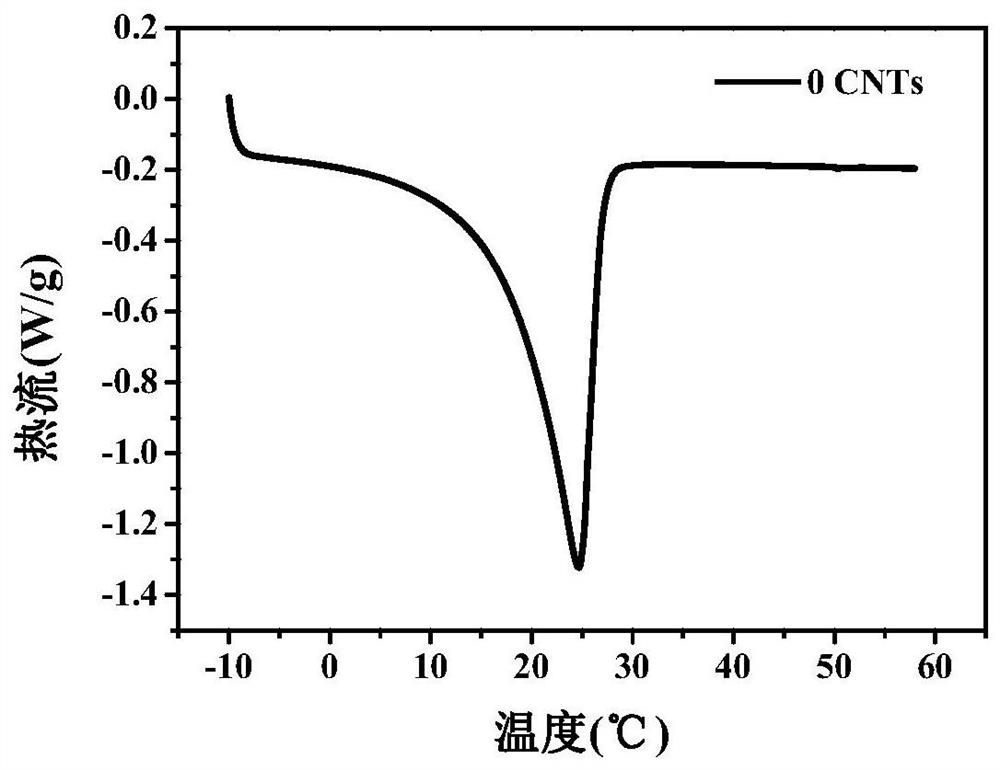
[0039] Embodiment 1 (with hydroxylated carbon nanotubes as nucleating agent)
[0040] (1) Dissolve anhydrous calcium chloride and anhydrous magnesium chloride in deionized water, prepare a nearly saturated solution at 60-100°C, carry out suction filtration and purification while it is hot, let it stand at room temperature for 24 hours, and remove the liquid by suction filtration to obtain high purity CaCl 2 ·6H 2 O, MgCl 2 ·6H 2 O crystals;
[0041] (2) Weigh 22.5g and 7.5g of CaCl respectively 2 ·6H 2 O and MgCl 2 ·6H 2 O crystals are placed in a beaker, heated to 60-100°C, and kept for 10 minutes to completely melt the mixture;
[0042] (3) Magnetically stir the system prepared in step (2) at 60-100° C. for 1-3 h, add 0.15 g of hydroxyethyl cellulose as a thickener and 0.75 g of hydroxylated carbon nanotubes as a nucleating agent during the stirring process, Ultrasonic dispersion at 40-50°C for 1-2 hours, until the system is evenly mixed, and finally MgCl 2 ·6H 2 ...
Embodiment 2
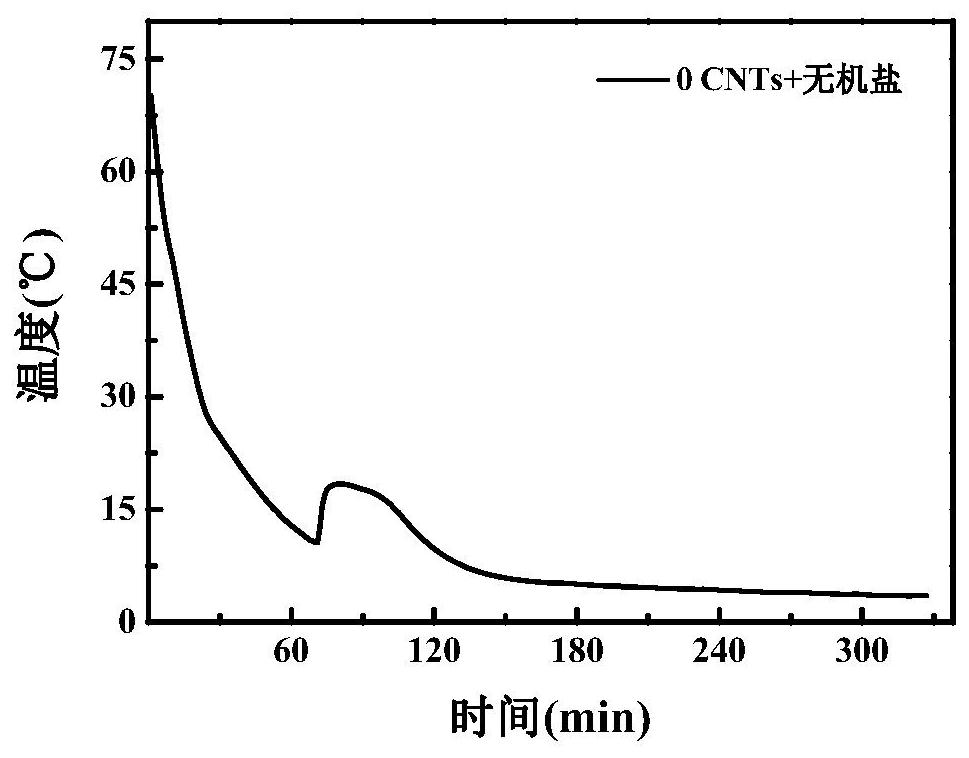
[0044] Embodiment 2 (with SrCl 2 ·6H 2 O, SrCO 3 as a nucleating agent)
[0045] (1) Dissolve anhydrous calcium chloride and anhydrous magnesium chloride in deionized water, prepare a nearly saturated solution at 60-100°C, carry out suction filtration and purification while it is hot, let it stand at room temperature for 24 hours, and remove the liquid by suction filtration to obtain high purity CaCl 2 ·6H 2 O, MgCl 2 ·6H 2 O crystals;
[0046] (2) Weigh 22.5g and 7.5g of CaCl respectively 2 ·6H 2 O and MgCl 2 ·6H 2 O crystals are placed in a beaker, heated to 60-100°C, and kept for 10 minutes to completely melt the mixture;
[0047] (3) Magnetically stir the system prepared in step (2) at high temperature for 2 hours, add 0.15 g of thickener during the stirring process, and disperse with high-temperature ultrasonic for 1 hour until the system is evenly mixed;
[0048] (4) Weigh again 0.9g of SrCl 2 ·6H 2 O and 0.3 g of SrCO 3 As an inorganic salt nucleating age...
Embodiment 3
[0050] Embodiment 3 (with hydroxylated carbon nanotubes as nucleating agent, and change the add-on of hydroxylated carbon nanotubes)
[0051] (1) Dissolve anhydrous calcium chloride and anhydrous magnesium chloride in deionized water, prepare a nearly saturated solution at 60-100°C, carry out suction filtration and purification while it is hot, let it stand at room temperature for 24 hours, and remove the liquid by suction filtration to obtain high purity CaCl 2 ·6H 2 O, MgCl 2 ·6H 2 O crystals;
[0052] (2) Weigh 22.5g and 7.5g of CaCl respectively 2 ·6H 2 O and MgCl 2 ·6H 2 O crystals are placed in a beaker, heated to 60-100°C, and kept for 10 minutes to completely melt the mixture;
[0053] (3) Magnetically stir the system prepared in step (2) at 60-100°C for 1-3 hours, add 0.15g hydroxyethyl cellulose as a thickener and 0.00g, 0.15g, 0.30g, 0.45g during the stirring process, 0.60g, 0.75g of hydroxylated carbon nanotubes are used as nucleating agents, ultrasonicall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com