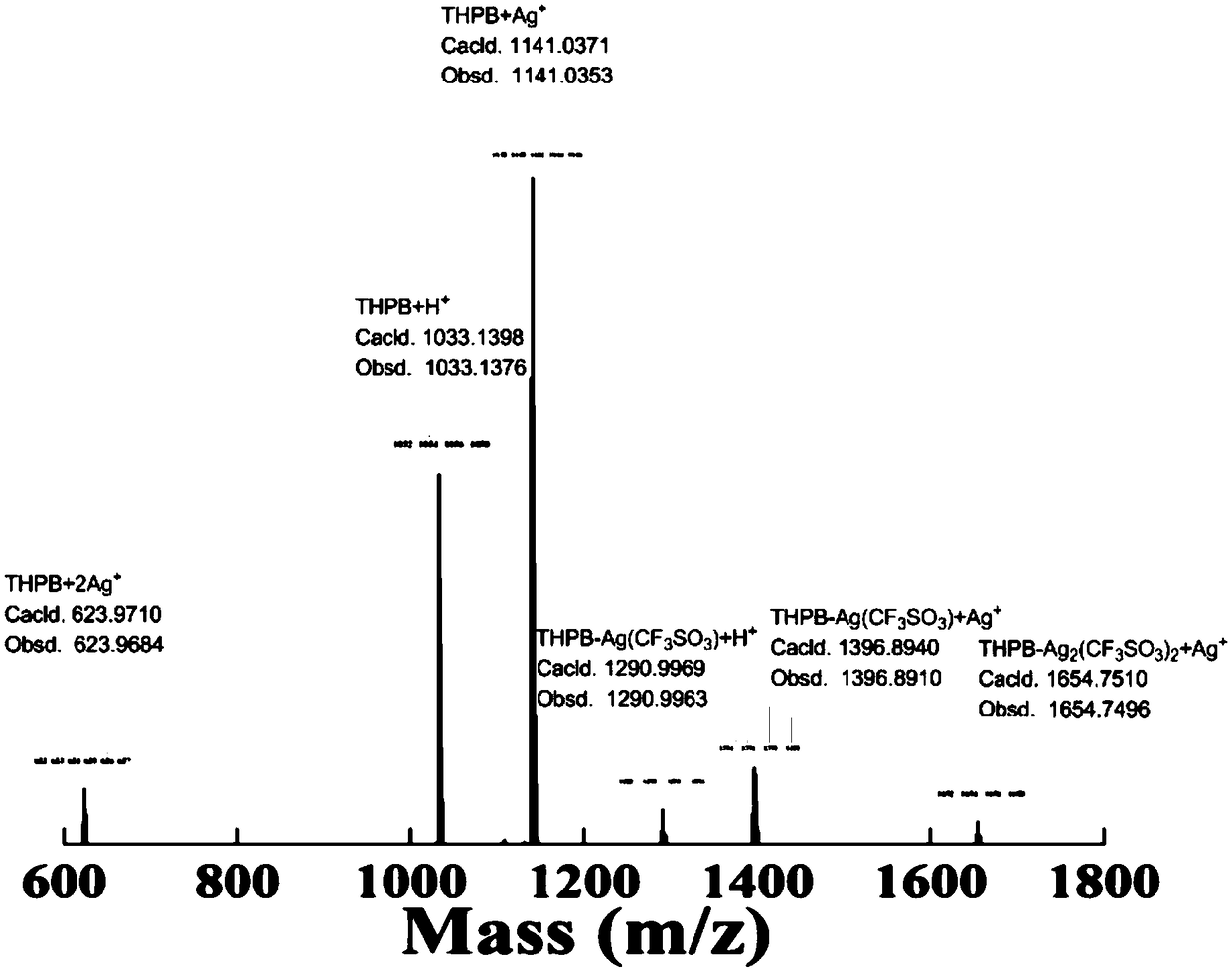
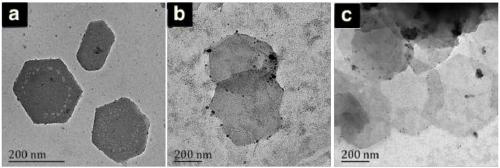
Organic microporous material with metal coordination containing heteroatoms and preparation and application thereof
A technology of microporous materials and metal coordination, applied in metal coordination heteroatom-containing organic microporous materials and their preparation and application fields, can solve problems such as poor thermal stability, and achieve uniform pore size, uniform pore size distribution, and large nitrogen adsorption. amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of compound 3:
[0047] Under argon protection, add PdCl to a dry two-necked flask equipped with magnetic stirring 2 (PPh 3 ) 2 (1.71g, 2.44mmol), cuprous iodide (0.77g, 4mmol), 2-bromo-4-iodopyridine (11.5g, 40.4mmol) and 50mL of triethylamine, stirred in an oil bath at 50°C, and Add trimethylsilylacetylene (5.68mL, 40.4mmol) into the reaction vial with a syringe, react at this temperature for 24 hours, TLC traces the completion of the reaction, and stops the reaction. Cool to room temperature, wash with water, salt, anhydrous MgSO 4 After drying and suction filtration, the solvent was distilled off, dissolved in dichloromethane, and separated and purified by column chromatography (stationary phase: silica gel; developer: dichloromethane:petroleum ether=1:2) to obtain 5.52 g of a colorless liquid. Yield 75%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ8.31(d,J=5.1Hz,1H),7.52(s,1H),7.25(d,J=5.0Hz,1H),0.26(s,9H). 13 C NMR (101MHz, CDCl 3 )δ150.32, 142.81, 134...
Embodiment 2
[0050] Preparation of compounds 4 and 5:
[0051] Under argon protection, add compound 3 (5.52g, 30.33mmol), tetrabutylammonium fluoride (7.93g, 30.33mmol), and 50mL of newly distilled tetrahydrofuran solution into a 100mL two-necked flask, and stir for 30 minutes at room temperature ; Then add 10mL saturated NH 4 Cl solution and continued to stir for 5 minutes. Stop the reaction, evaporate the solvent, dichloromethane extraction, anhydrous MgSO 4 After drying and suction filtration, the solvent was distilled off to obtain compound 4 as a colorless oil. Since compound 4 was unstable, it was directly reacted in the next step. In a two-necked flask containing colorless oily compound 4, add 2-bromo-4-iodopyridine (8.61g, 30.33mmol), PdCl 2 (PPh 3 ) 2 (2.08g, 1.82mmol), cuprous iodide (3.48g, 3.03mmol), triethylamine (50mL). Stir in an oil bath at 50° C., react for 24 hours, and stop the reaction after tracking the completion of the reaction by TLC. After cooling to room te...
Embodiment 3
[0054] Preparation of Compound 6:
[0055] Under argon protection, into a 100mL two-necked flask, add compound 5 (7.38g, 21.83mmol), 2-thiophene boronic acid (5.58g, 43.66mmol), Pd(PPh 3 ) 4 (1.50 g, 1.31 mmol), potassium carbonate in THF (2M, 30 mL) was added. Heated to reflux, reacted for 24 hours, TLC tracked that the reaction was complete, and stopped the reaction. After cooling to room temperature, the mixture was extracted with dichloromethane (70mL×3), the organic layer was washed with water (50mL×3), anhydrous MgSO 4 dry. The solvent was distilled off, dichloromethane was dissolved, and then separated and purified by column chromatography (eluent: dichloromethane) to obtain 5.85 g of white solid with a yield of 78%.
[0056] 1 H NMR (400MHz, CDCl 3 )δ8.61(d, J=5.0Hz, 2H), 7.80(s, 2H), 7.67(d, J=3.1Hz, 2H), 7.46(d, J=5.0Hz, 2H), 7.30-7.26( m,2H),7.15(t,J=4.3Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ149.57, 145.24, 138.19, 134.19, 129.03, 127.41, 125.63, 123.79, 118.64...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com