Fluorene multifunctional photoinitiator and preparation method and application thereof
A photoinitiator, multifunctional technology, applied in the field of photocuring, can solve the problem of low quantum absorption rate at long wavelengths, achieve high quantum absorption rate, improve initiation efficiency, and improve the effect of quantum absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
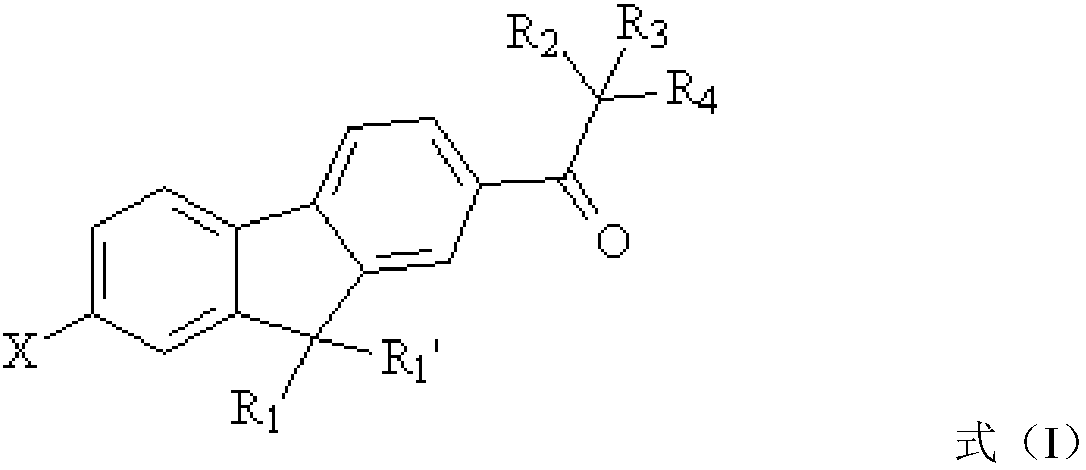
[0044] Another aspect of the present application also provides a preparation method of the above-mentioned fluorene-based polyfunctional photoinitiator, the preparation method comprising: bromination reaction: reacting raw material a, bromination reagent and the first organic solvent with bromination reaction to obtain Intermediate product b, raw material a has the structure shown in formula (II),
[0045]
[0046] The intermediate product b has the structure of formula (Ⅲ),
[0047]
[0048] Substitution reaction: use the intermediate product b, a substitution reagent and a second organic solvent to undergo a substitution reaction to obtain a fluorene-based multifunctional photoinitiator.
[0049] According to the conventional understanding of those skilled in the art, the substituent group R in the structural formula shown in formula (II) and formula (III) 1 , R 2 , R 3 , R 4 And X has the same structure as the corresponding group in formula (I). The chemical reac...
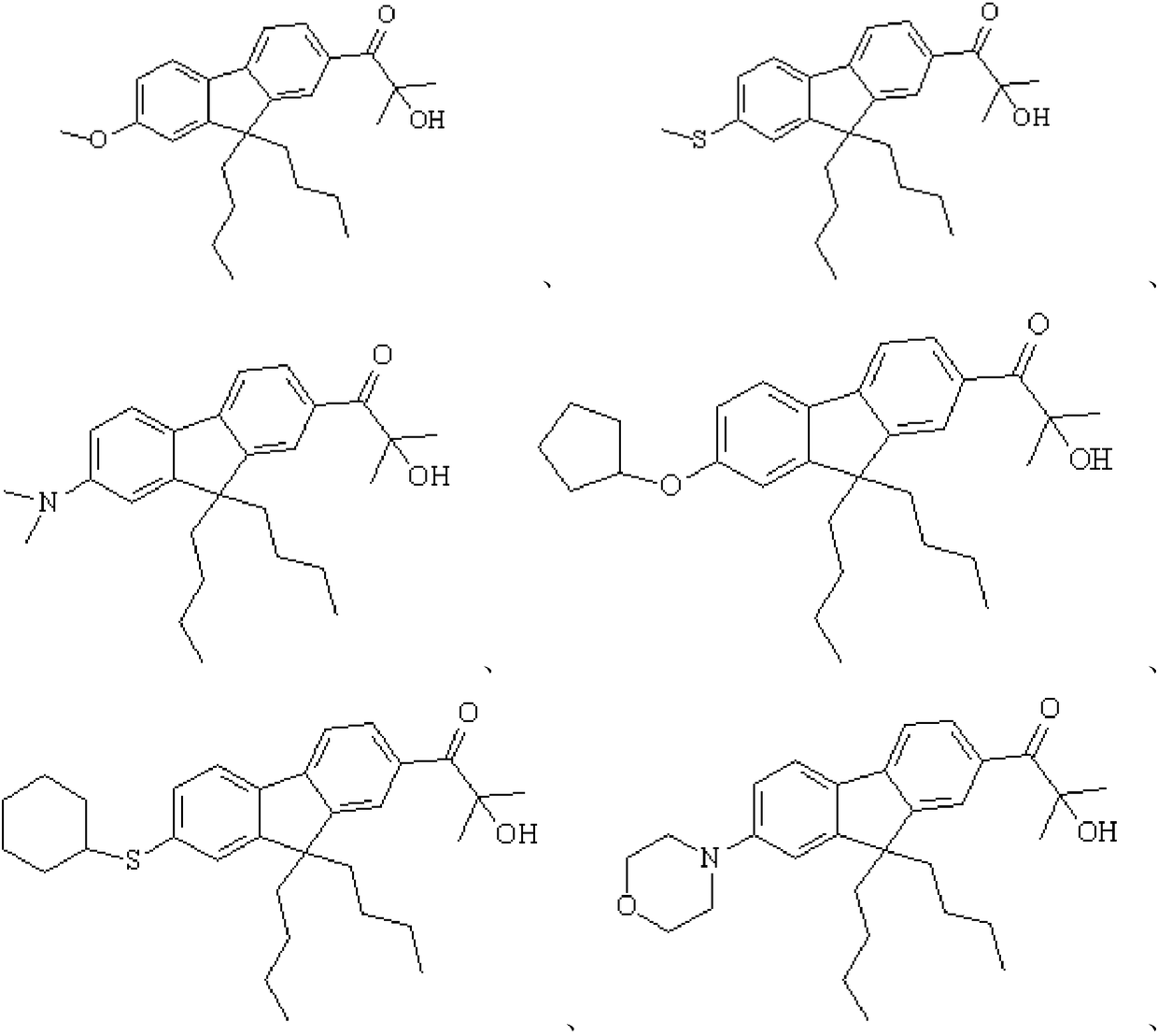
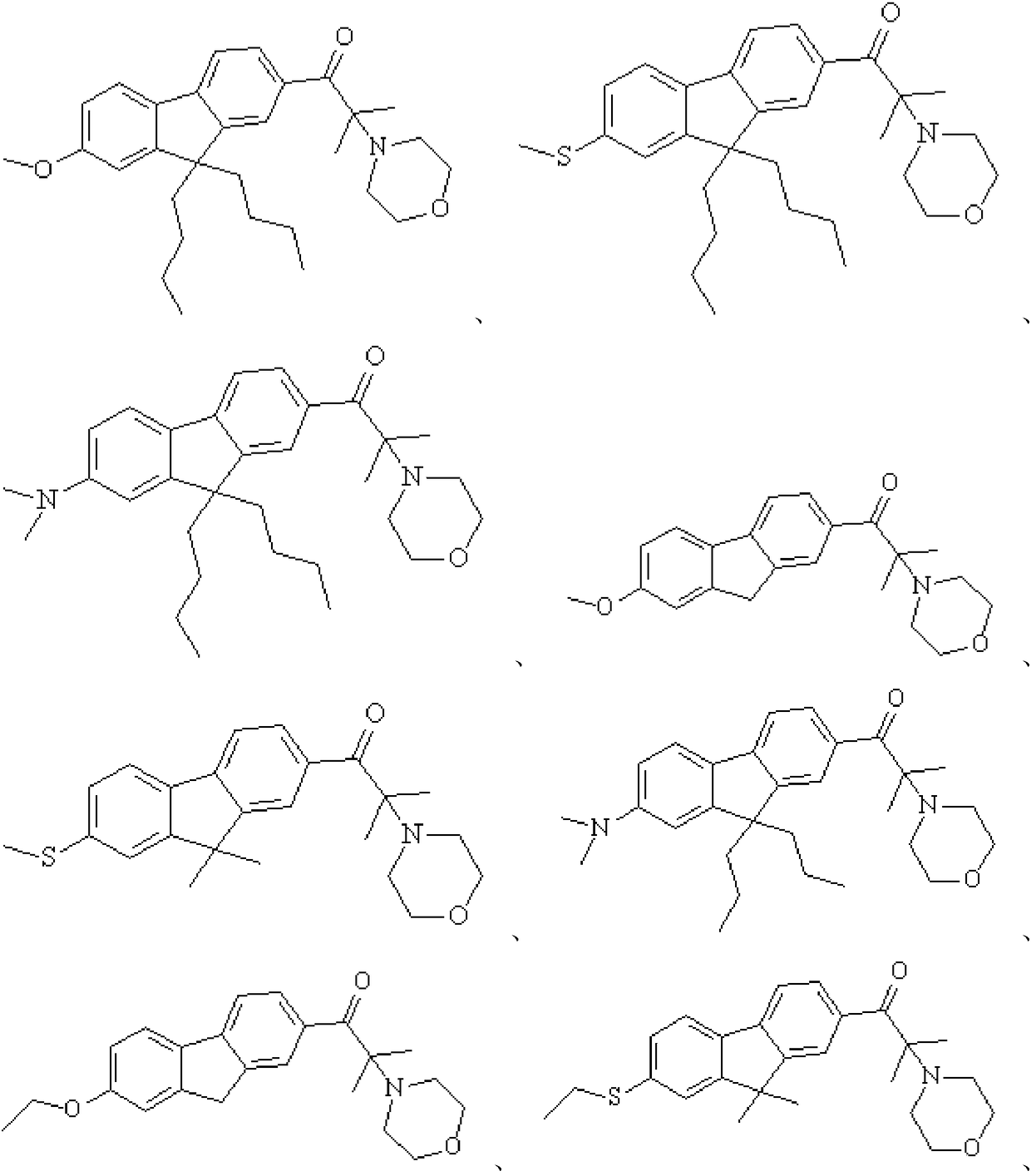
preparation Embodiment 1
[0078] (1) Substitution reaction: Preparation of intermediate 1a.
[0079] Add 36.5g of raw material 1a, 18g of N-bromosuccinimide, and 100mL of propylene carbonate to a 500mL four-neck flask, stir at room temperature, follow the reaction of the liquid phase until the raw materials no longer change, and then slowly pour the materials into 1000g to Stirring while adding in deionized water, a large amount of solids precipitated, suction filtered, washed with water, and absolute ethanol to obtain 37.2g of intermediate 1a. The synthetic route is as follows:
[0080]
[0081] The structural characterization data of the intermediate product 1a are as follows: 1 H-NMR (CDCl 3 , 500MHz): 0.9146~1.0002(6H,t), 1.2878~1.3328(8H,m), 1.4844(6H,s), 1.8754~2.1045(5H,m), 7.5801~8.0837(6H,m).
[0082] MS(m / z):444(M+1) + .
[0083] (2) Substitution reaction: preparation of compound 1.
[0084]Add 22.2g of intermediate 1a, 50mL of methanol, and 14.0g of sodium methoxide into a 500mL fou...
preparation Embodiment 2
[0088] Step substitution reaction: Preparation of intermediate 2a.
[0089] Add 35.0g of raw material 2a, 18g of N-bromosuccinimide, and 100mL of propylene carbonate to a 500mL four-neck flask, stir at room temperature, follow the reaction of the liquid phase until the raw materials no longer change, and then slowly pour the materials into 1000g to In deionized water, stirring while adding, a large amount of solids precipitated out, suction filtered, washed with water, and absolute ethanol to obtain 36.8g of intermediate 2a. The synthetic route is as follows:
[0090]
[0091] The structural characterization data of the intermediate product 2a are as follows: 1 H-NMR (CDCl 3 , 500MHz): 1.3629 (6H, s), 1.6578 (6H, s), 2.3899-2.3835 (4H, t), 3.6703-3.6801 (4H, t), 7.5481-7.8997 (6H, m).
[0092] MS(m / z):429(M+1) + .
[0093] (2) Substitution reaction: preparation of compound 2.
[0094] Add 21.4g of intermediate 2a, 50mL of xylene, and 14.0g of sodium methoxide to a 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com