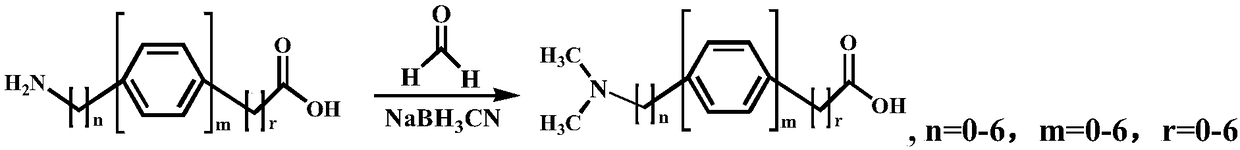
Isotope derivatization reagent for labeling amino/phenolic hydroxyl and synthesis method thereof
A technology for derivatization reagents and synthesis methods, which is applied in the fields of organic chemistry, measuring devices, instruments, etc., can solve the problems of low commercialization degree and high price of isotope internal standard reagents, and achieve the effect of saving costs, improving efficiency and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one (corresponding reagent series one)
[0031] Select p-aminophenylacetic acid, NaBH3CN and CH 2 O is raw material, and concrete steps are:
[0032] a) Use a 1.5mL centrifuge tube to directly weigh 10mg (0.066mmol) p-aminophenylacetic acid;
[0033] b) Use 500 μL of ultrapure water to dissolve p-aminophenylacetic acid, which can be dissolved by shaking or ultrasound;
[0034] c) Add 7.4% CH2 O 100μL, mix well during the shaking period of 10s;
[0035] d) Add 0.6mol / L catalyst NaBH 3 CN 150μL, mix well during shaking for 10s;
[0036] e) Use 0.5% formic acid (FA) to control the pH of the solution to 5, and dilute to 1 mL with ultrapure water;
[0037] f) React for 1 h at room temperature under the condition of 500 rpm. Through above-mentioned six steps operation, can obtain p-dimethylaminophenylacetic acid altogether (reaction equation is as follows);
[0038]
[0039] g) Transfer the dimethylation reaction product of the first step into a clean 25m...
Embodiment 2
[0046] Embodiment two (corresponding reagent series one)
[0047] Use p-aminophenylbutyric acid, NaBH 3 CN and CD 2 O is a raw material, and its concrete steps are:
[0048] a) Use a 1.5mL centrifuge tube to directly weigh 10mg p-aminobenzenebutyric acid;
[0049] b) Use 500 μL of ultra-pure water to dissolve p-aminophenylbutyric acid, which can be dissolved by shaking or ultrasound;
[0050] c) Add 20% CH 2 O 30μL, shake well for 10s;
[0051] d) Add 0.6mol / L catalyst NaBH 3 CN 150μL, mix well during shaking for 10s;
[0052] e) Use 0.5% formic acid (FA) to control the pH of the solution to 6, and dilute to 1 mL with ultrapure water;
[0053] f) React for 1 h at room temperature under the condition of 500 rpm. Through above-mentioned six steps operation (reaction equation is as follows);
[0054]
[0055] g) Transfer the dimethylation reaction product of the first step into a clean 25mL round bottom flask, and directly use a rotary evaporator to remove water (rota...
Embodiment 3
[0062] Embodiment three (corresponding reagent series one)
[0063] Select 4-(2-aminoethyl)benzoic acid, NaBD 3 CN and CH 2 O is a raw material, and its concrete steps are:
[0064] a) Use a 1.5mL centrifuge tube to directly weigh 10mg of 4-(2-aminoethyl)benzoic acid;
[0065] b) Use 500 μL of ultrapure water to dissolve 4-(2-aminoethyl)benzoic acid, which can be dissolved by shaking or ultrasound;
[0066] c) Add 7.4% CH 2 O 140μL, mixed well during the shaking period of 10s;
[0067] d) Add 0.6mol / L catalyst NaBD 3 CN 160μL, mix well during the shaking period of 10s;
[0068] e) Use 0.5% formic acid (FA) to control the pH of the solution to 6, and dilute to 1 mL with ultrapure water;
[0069] f) React for 1 h at room temperature under the condition of 500 rpm. Through above-mentioned six steps operation (reaction equation is as follows);
[0070]
[0071] g) Transfer the dimethylation reaction product of the first step into a clean 25mL round bottom flask, and di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com