Preparation method of oxazole derivative
A technology of derivatives and oxazoles, applied in the field of preparation of oxazole derivatives, can solve the problems of poor atom economy and harsh reaction conditions, and achieve the effects of scientific and reasonable synthesis method, easy purification and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
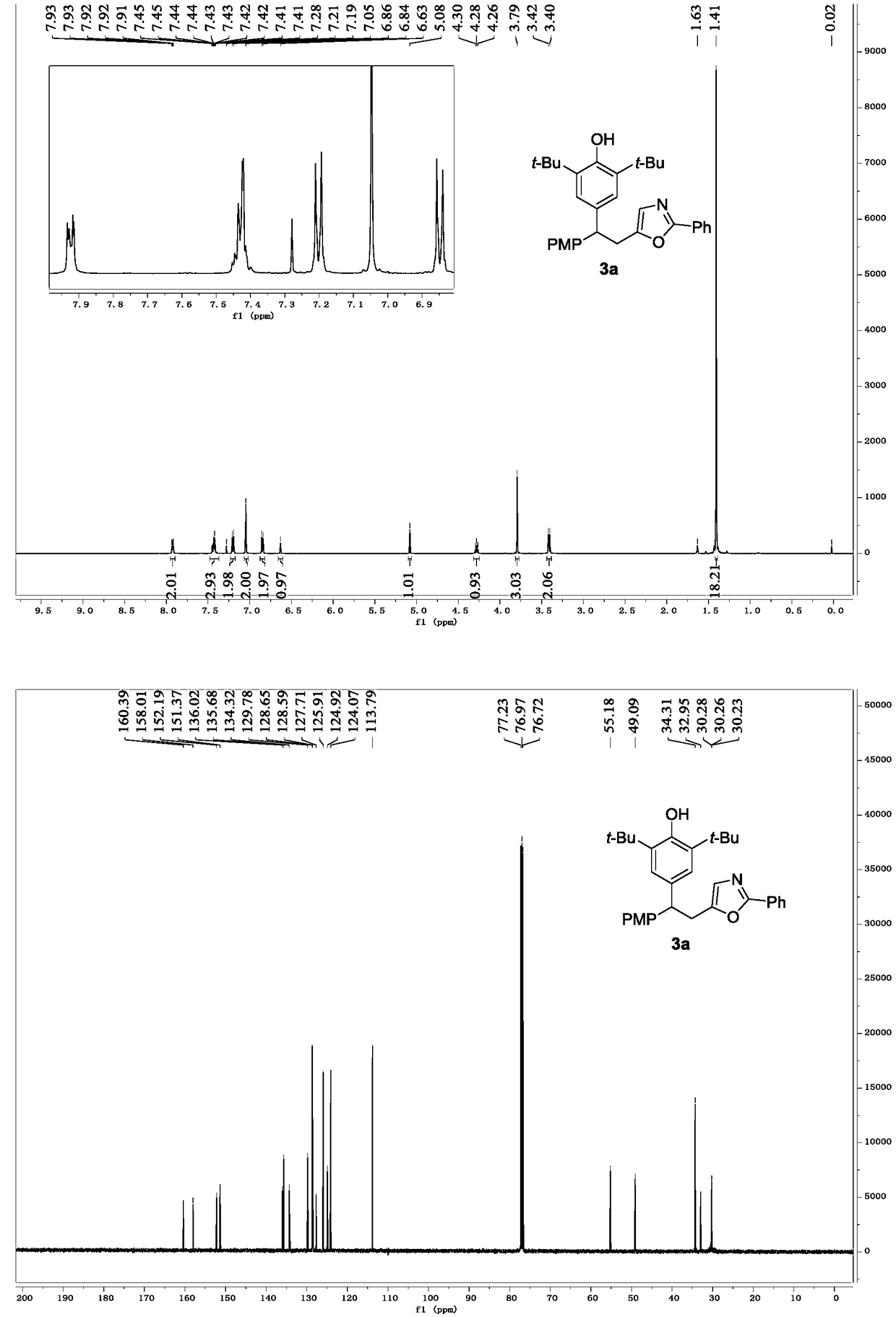
[0022] Embodiment 1: the preparation of oxazole derivative 3a
[0023]
[0024] Add p-methylenebenzoquinone 1a (0.5mmol, 162.2mg), N-propargylbenzamide 2a (0.75mmol, 119.4mg) and InCl to a 25mL single-necked flask 3 (0.05 mmol, 11.1 mg). Add 1,2-dichloroethane (2.5 mL), stir in an oil bath at 70° C., and react for 3 hours. After the reaction is complete, cool to room temperature, remove the solvent with a rotary evaporator, and separate the residue through column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=20 / 1), and the solid obtained by rotary evaporation is washed with n-hexane Washing twice gave the oxazole derivative 3a as a white solid with a yield of 94%.
[0025] Spectral analysis data 3a:
[0026] 1 H NMR (CDCl 3 ,500MHz)δ7.95–7.89(m,2H),7.48–7.37(m,3H),7.03(dd,J=8.6,8.7Hz,4H),7.05(s,2H),6.63(s,1H) ,5.08(s,1H,missing after deuteriation),4.28(t,J=8.0Hz,1H),3.79(s,3H),3.41(d,J=8.0Hz,2H),1.41(s,18H); 13 C NMR (CDCl 3 ,125MHz)δ160.39...
Embodiment 2
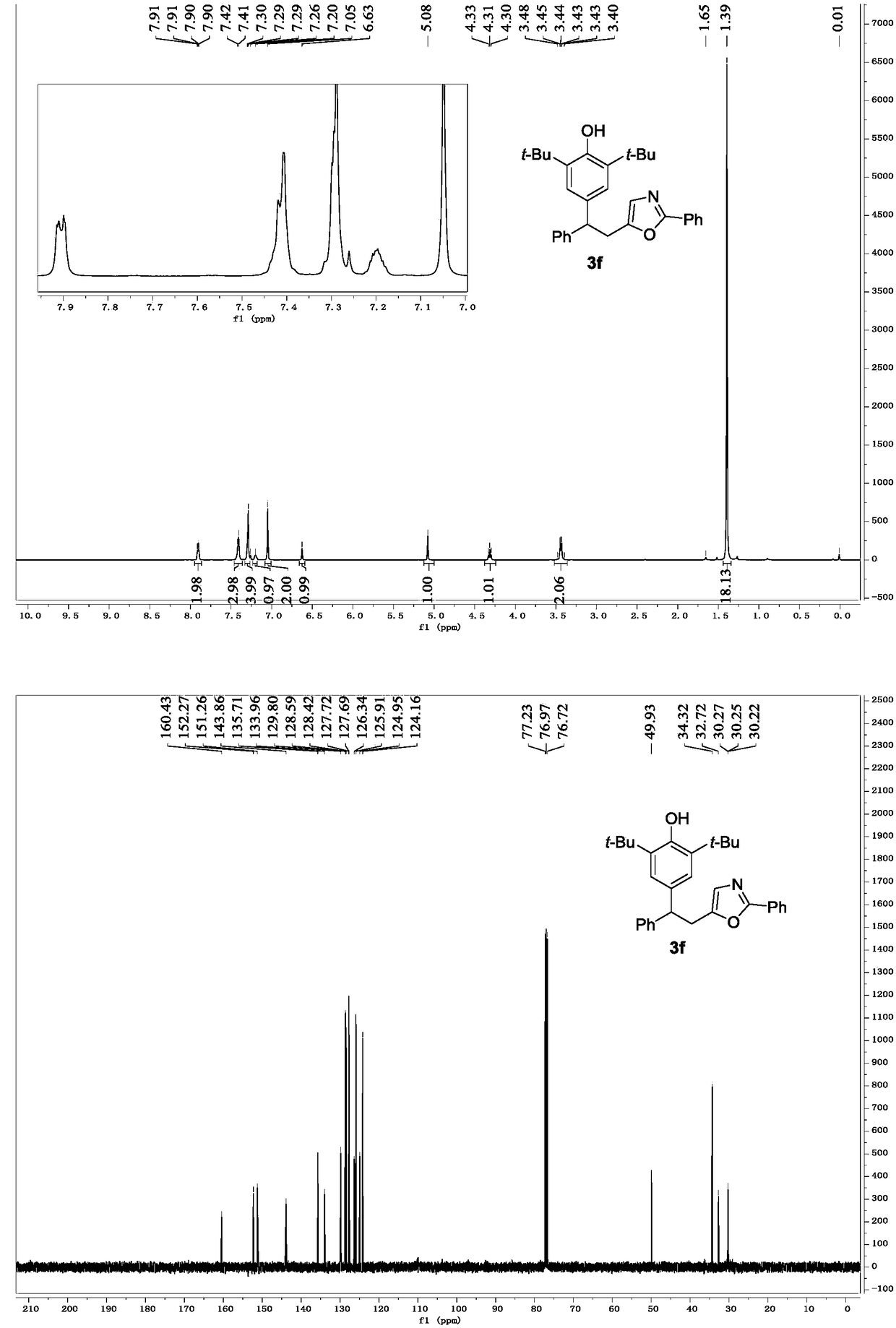
[0028] Replace 2a in Example 1 with 2b, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0029]
[0030] Spectrum analysis data 3b:
[0031] 1 H NMR (CDCl 3 ,500MHz)δ7.65(dd,J=8.5,8.6Hz,4H),7.00(dd,J=8.6,8.7Hz,4H),7.01(s,2H),6.61(s,1H),5.06(s ,1H),4.24(t,J=8.0Hz,1H),3.77(s,3H),3.38(d,J=7.9Hz,2H),1.38(s,18H); 13 C NMR (CDCl 3 ,125MHz)δ159.52,158.08,152.21,151.77,135.95,135.76,134.24,131.83,128.62,127.38,126.64,125.11,124.13,124.03,113.82,77.20,76.94,76.69,55.17,49.12,34.30,32.92,30.28,30.26 ,30.23; HRMS(ESI)m / zcalcd for C 32 h 37 NO 3 Br + [M+H] + 562.1957, found 562.1956.
Embodiment 3
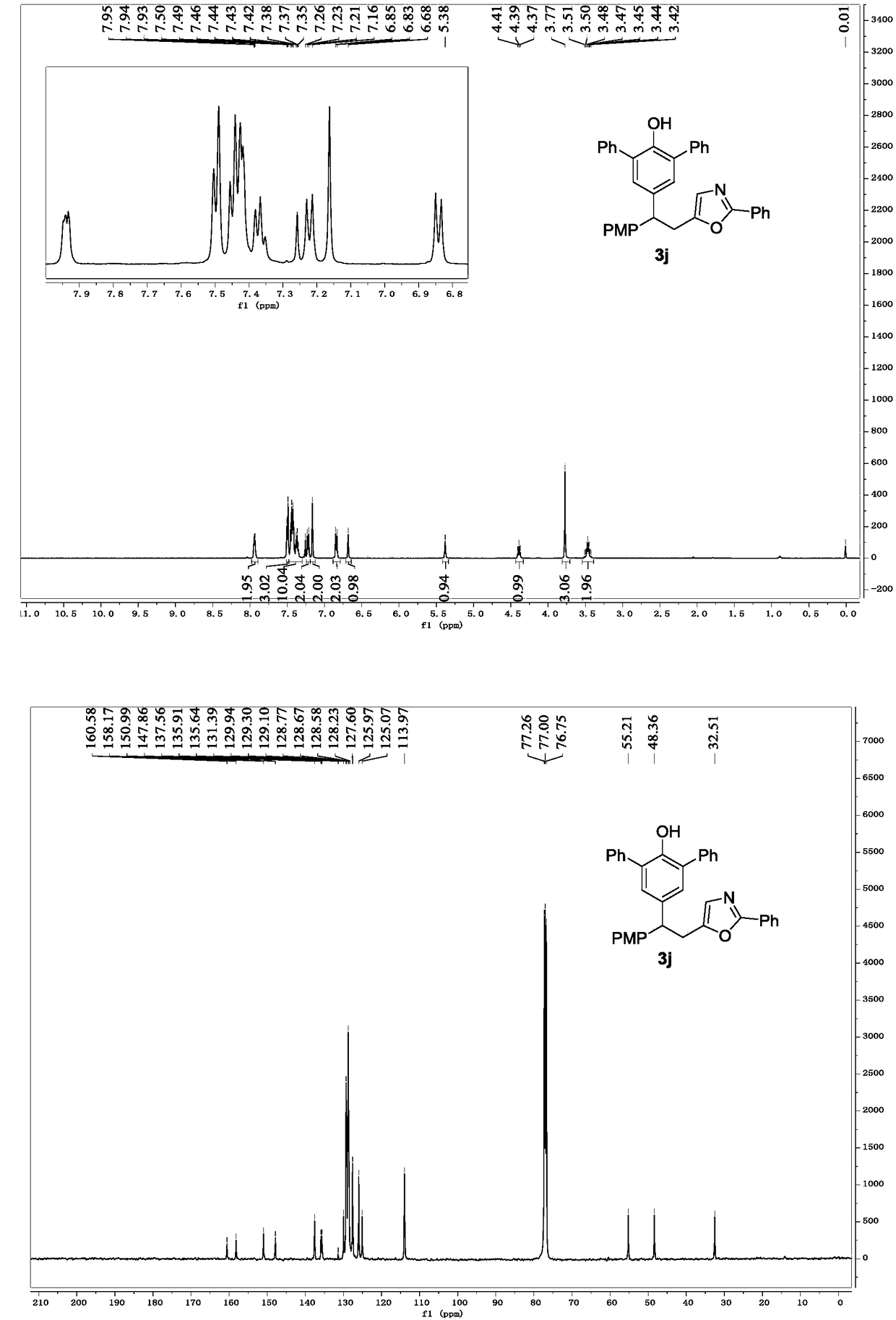
[0033] Replace 2a in Example 1 with 2c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0034]
[0035] Spectrum analysis data 3c:
[0036] 1 H NMR (CDCl 3 ,500MHz)δ7.51(dd,J=8.1,7.9Hz,4H),7.01(dd,J=8.5,8.5Hz,4H),7.03(s,2H),6.59(s,1H),5.07(s ,1H),4.26(t,J=7.9Hz,1H),3.77(s,3H),3.38(d,J=7.9Hz,2H),2.39(s,3H),1.39(s,18H); 13 C NMR (CDCl 3 ,125MHz)δ160.61,157.99,152.18,151.00,139.97,136.07,135.65,134.36,129.30,128.66,125.87,125.05,124.75,124.07,113.77,77.23,76.97,76.72,55.17,49.06,34.31,32.94,30.25,21.43 ;HRMS(ESI)m / zcalcd for C 33 h 40 NO 3 + [M+H] + 498.3008,found 498.3001.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com