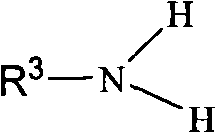
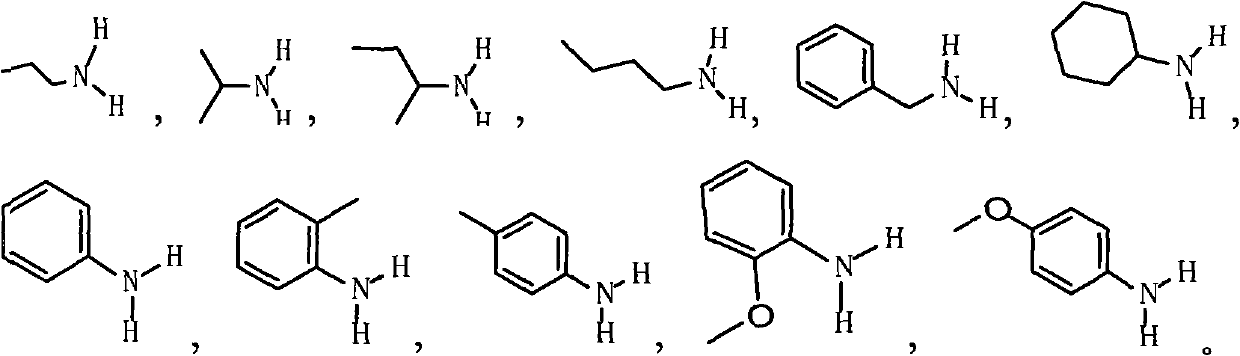
Method for synthesizing 1,2,3,4-tetrahydropyridine derivatives from chain 1,3-dicarbonyl compound in one-pot method
A technology of dicarbonyl compounds and tetrahydropyridines, applied in the field of synthesis of organic intermediates, achieving high yield, mild conditions, and scientific and reasonable synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of N-n-propyl-2-methyl-3,5,5-triacetyl-1,2,3,4-tetrahydropyridine:
[0024] At room temperature, add 3 mmol (315 μl) of acetylacetone, 1 mmol (82 μl) of n-propylamine, 2 mmol of formaldehyde (157 μl of 37% aqueous formaldehyde solution) and 1 mL of ethanol as a solvent in a 5 ml small flask, and react with magnetic stirring for 6 hours at room temperature. After the reaction was completed, it was concentrated by a rotary evaporator, and the residue was separated by silica gel thin-layer chromatography using 1:1 petroleum ether / ethyl acetate as a developing solvent. Pure N-n-propyl-2-methyl-3,5,5-triacetyl-1,2,3,4-tetrahydropyridine (purity>98%, red viscous liquid) was obtained. The isolated yield was 74%. The nuclear magnetic analysis data of this compound are as follows:
[0025] 1 H NMR δ=0.74(t, 3H, CH 3 ), 1.44 (m, 2H, CH 2 ), 2.00(s, 6H, 2CH 3 ), 2.05(s, 3H, CH 3 ), 2.17 (s, 3H, CH 3 ), 2.72 (s, 2H, CH 2 ), 3.08(t, 2H, CH 2 ), 3.39 (s...
Embodiment 2
[0027] Example 2: Synthesis of N-isopropyl-2-methyl-3,5,5-triacetyl-1,2,3,4-tetrahydropyridine:
[0028] At room temperature, add 2mmol (210μl) acetylacetone, 1mmol (86μl) isopropylamine, 2mmol formaldehyde (157μl of 37% formaldehyde aqueous solution) to a 5ml small flask, and 2mL of DMF as a solvent, magnetic stirring reaction at room temperature for 4 hours, and 1 : 1 petroleum ether / ethyl acetate as developing solvent, separated by silica gel thin-layer chromatography to obtain pure product N-isopropyl-2-methyl-3,5,5-triacetyl-1,2,3,4 - Tetrahydropyridine (purity > 98%, red viscous liquid). Isolated yield 78%.
[0029] The NMR data of this compound are as follows.
[0030] 1 H NMR δ=1.04(s, 3H, 1CH 3 ), 1.06(s, 3H, 1CH 3 ), 2.03(s, 6H, 2CH 3 ), 2.08(s, 3H, 1CH 3 ), 2.22(s, 3H, 1CH 3 ), 2.75(s, 2H, 1CH 2 ), 3.28(s, 2H, 1CH 2 ), 4.06(m, 1H, 1CH)
[0031] 13 C NMR δ = 16.7, 19.8, 26.2, 30.1, 30.2, 43.8, 48.1, 64.0, 101.7, 155.4, 195.4, 204.2.
Embodiment 3
[0032] Example 3: Synthesis of N-n-propyl-2-methyl-5-acetyl-1,2,3,4-tetrahydropyridine-3,5-dioic acid diethyl ester:
[0033] At room temperature, add 3mmol (381μl) ethyl acetoacetate, 1mmol (82μl) n-propylamine, 2.5mmol formaldehyde (196μl of 37% formaldehyde aqueous solution) and 2mL of methanol as a solvent in a 5ml small flask, and magnetically stir the reaction for 7 hours at room temperature , using 5:1 petroleum ether / ethyl acetate as a developing solvent, separated by silica gel thin layer chromatography to obtain pure product N-n-propyl-2-methyl-5-acetyl-1,2,3,4-tetra Hydropyridine-3,5-dioic acid diethyl ester (purity>98%, yellow viscous liquid). Isolated yield 83%.
[0034] The NMR data of this compound are as follows:
[0035] 1 H NMR δ=0.89(t, 3H, 1CH 3 ), 1.265 (m, 6H, 2CH 3 ), 1.583 (m, 2H, 1CH 2 ,), 2.23(s, 3H, 1CH 3 ), 2.36(s, 3H, 1CH 3 ), 2.92 (m, 2H, 1CH 2 ), 3.21(t, 2H, 1CH 2 ), 3.44(t, 2H, 1CH 2 ), 4.18 (m, 4H, 2CH 2 )
[0036] 13 C NMR δ = 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com