A kind of diclazuril enantiomer chiral chromatographic separation analysis method
A technology of separation analysis and chiral chromatography, which is applied in the field of chiral chromatography separation and analysis of diclazuril enantiomers to achieve good separation effect and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~14
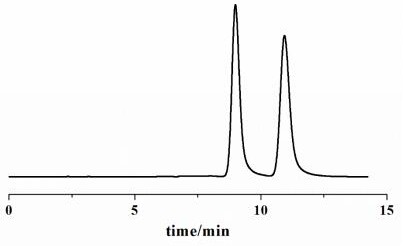
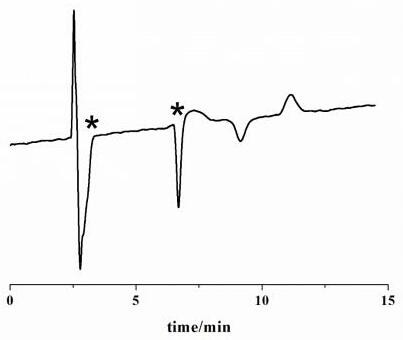
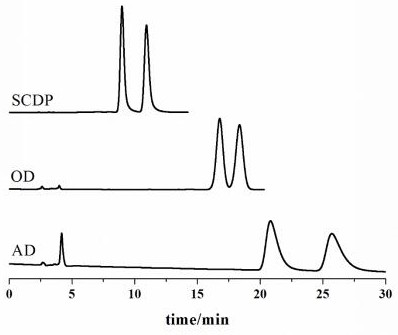
[0034] The chiral chromatographic separation and analysis method of diclazuril enantiomers in Examples 1-14
[0035] The specific steps of the chiral chromatographic separation and analysis method of diclazuril enantiomers provided in each example are as follows: dissolve diclazuril in N,N-dimethylformamide and prepare 5.0 mg·mL -1 solution, and then diluted with ethanol to 0.5 mg·mL -1 , after controlling the conditions such as mobile phase composition, mobile phase flow rate and chromatographic column temperature, the chromatographic separation of diclazuril was carried out.
[0036] In the chiral chromatographic separation and analysis methods of Examples 1 to 14, the chromatograph used is Agilent 1200 series liquid chromatograph, the detector is Agilent 1260 type ultraviolet detector and German CHIRRALYSER-MP optical detector, and the chromatographic column is Guangzhou Research Institute. Produced by Chuang Biotechnology Development Co., Ltd. SCDP, OD and AD chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com