Diclazuril enantiomer chiral chromatographic separation analysis method
A technology for separation and analysis of diclazuril, applied in the field of chiral chromatographic separation, to achieve the effect of fast analysis speed and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~14
[0034] The chiral chromatographic separation and analysis method of diclazuril enantiomers in Examples 1-14
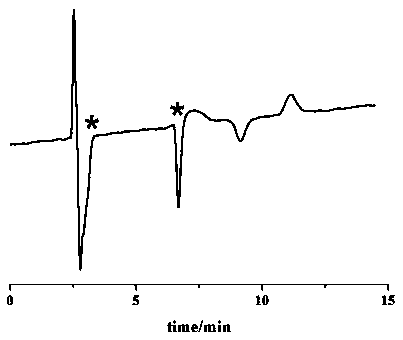
[0035] The specific steps of the chiral chromatographic separation and analysis method of diclazuril enantiomers provided in each example are as follows: dissolve diclazuril in N,N-dimethylformamide and prepare 5.0 mg·mL -1 solution, and then diluted with ethanol to 0.5 mg·mL -1 , after controlling the conditions such as mobile phase composition, mobile phase flow rate and chromatographic column temperature, the chromatographic separation of diclazuril was carried out.
[0036] In the chiral chromatographic separation and analysis methods of Examples 1 to 14, the chromatograph used is Agilent 1200 series liquid chromatograph, the detector is Agilent 1260 type ultraviolet detector and German CHIRRALYSER-MP optical detector, and the chromatographic column is Guangzhou Research Institute. Produced by Chuang Biotechnology Development Co., Ltd. SCDP, OD and AD chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com