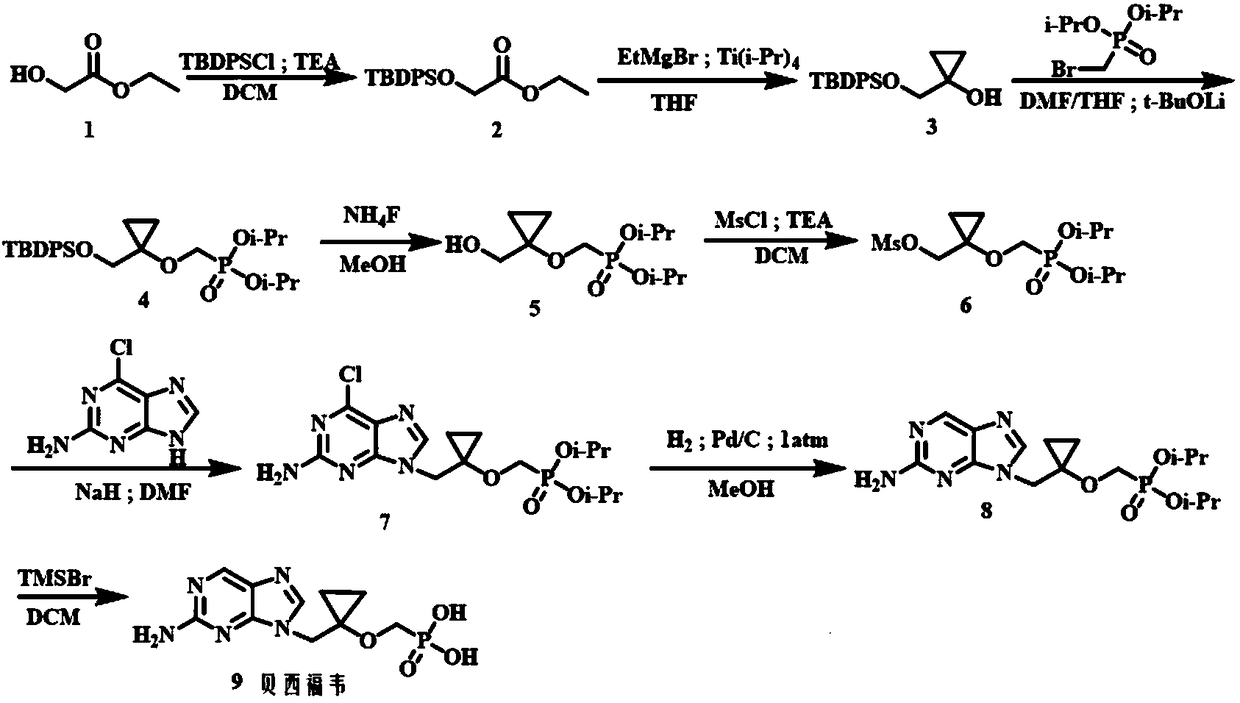
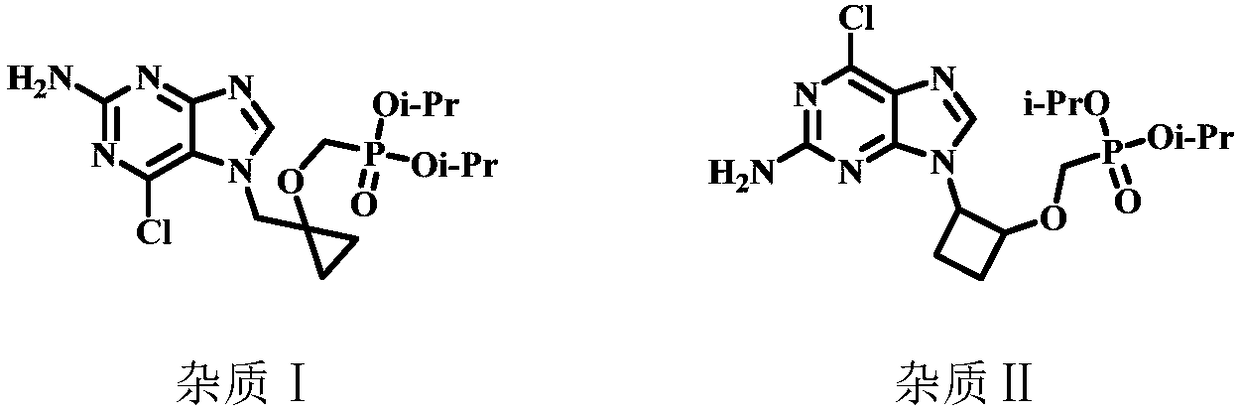
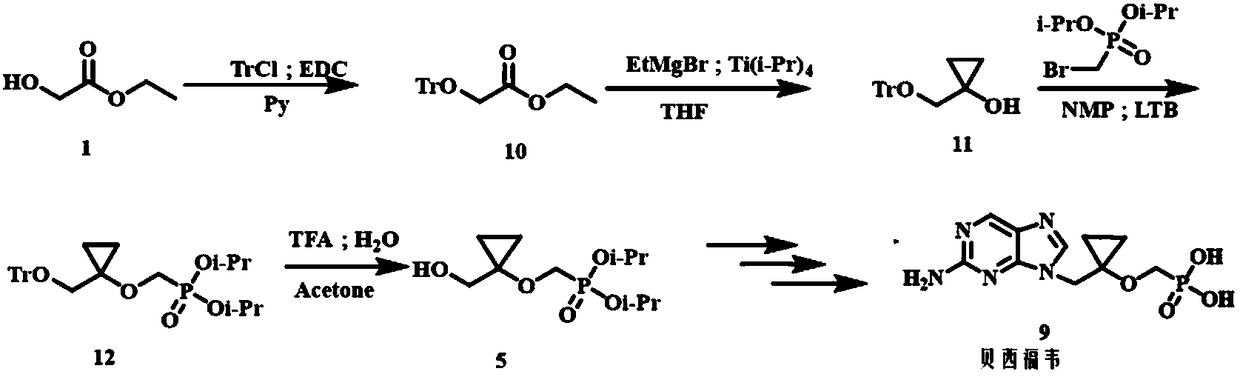
Method for preparing Besifovir
A compound, methyl technology, applied in the field of new intermediates, can solve the problems of increasing experimental costs and expensive prices, and achieve the effects of reducing the difficulty of purification, increasing yield, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] Step 1: Preparation of 2-((tert-butyl(diphenyl)silyl)oxy)ethyl acetate (2):
[0050] 1 (100.0g, 960.52mmol) and imidazole (77.3g, 1.15mol) were dissolved in dichloromethane (1.5L), and tert-butyldiphenylchlorosilane was slowly added dropwise with a constant pressure dropping funnel under ice-cooling conditions (264.0 g, 960.52 mmol). After the dropwise addition was completed, it was transferred to room temperature for 16 h. Filtrate, wash the filtrate with 1.0mol / L hydrochloric acid solution (300ml×2), wash the dichloromethane layer with saturated sodium bicarbonate solution (100ml×2), dry over anhydrous sodium sulfate, and concentrate to give white transparent liquid 2 (297.7g ,91%). ESI-MS(m / z):365.1564[M+Na] + ; 1 H NMR (300MHz, CDCl3) δ: 7.87~7.76 (m, 4H), 7.47 (q, J = 5.4Hz, 6H), 4.35 (s, 2H), 4.22 (q, J = 7.1Hz, 2H), 1.28 (t,J=7.1Hz,3H),1.21(s,9H); 13 CNMR (75MHz, CDCl3) δ: 171.2, 135.7, 132.9, 130.0, 127.9, 62.4, 60.7, 26.8, 19.4, 14.2.
[0051] Step 2: Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com