Method for catalytically synthesizing 4-fluorotetrahydropyran derivative by using ionic liquid of tetrafluoroboric acid as fluorine source
A fluorotetrahydropyran, ionic liquid technology, applied in the direction of organic chemistry, etc., to achieve the effect of safe and efficient reaction, mild reaction conditions and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
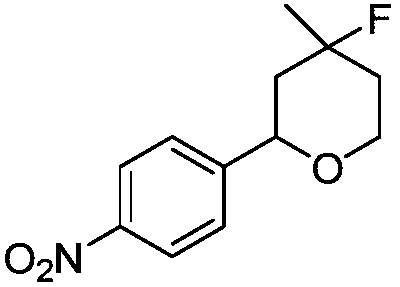
[0012] Synthesis of 4-fluoro-4-methyl-2-(4-nitrophenyl)-tetrahydropyran with the following structural formula
[0013]
[0014] 0.1511g (1.0mmol) p-nitrobenzaldehyde, 131μL (1.3mmol) 3-methyl-3-buten-1-ol, 0.0248g (0.1mmol) titanocene dichloride, 5-sulfosalicyl Add 0.07626g (0.3mmol) of acid, 0.4800g (2.0mmol) of 1-butyl-2,3-dimethylimidazolium tetrafluoroborate, 3mL of dry dichloromethane into the Shrek tube, and stir the reaction at room temperature After 5 hours, stop the reaction and separate by column chromatography to obtain solid 4-fluoro-4-methyl-2-(4-nitrophenyl)-tetrahydropyran with a yield of 83%, and the structural characterization data are: 1 H NMR (600MHz, CDCl 3 )δ8.15(d, J=8.8Hz, 4H), 7.48(d, J=8.6Hz, 4H), 4.75(d, J=2.1Hz, 1H), 4.73(d, J=2.1Hz, 1H) ,4.07–4.01(m,2H),3.90(td,J=11.6,2.7Hz,2H),2.07(ddt,J=13.7,9.3,2.1Hz,2H),1.83-1.73(m,4H),1.54 (ddd,J=38.3,14.1,11.8Hz,2H),1.41(s,3H),1.38(s,3H); 13 C NMR (151MHz, CDCl 3 )δ149.80(s), 147.29(s), 126.26(s), 123....
Embodiment 2
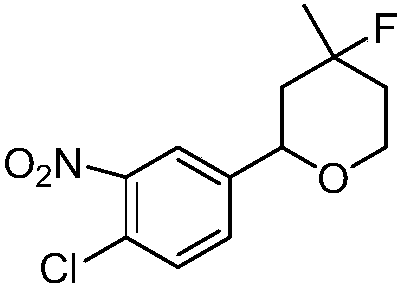
[0016] Synthesis of 2-(4-chloro-3-nitrophenyl)-4-fluoro-4-methyl-tetrahydropyran with the following structural formula
[0017]
[0018] In this example, the p-nitrobenzaldehyde in Example 1 is replaced with equimolar 4-chloro-3-nitrobenzaldehyde, and the other steps are the same as in Example 1 to obtain solid 2-(4-chloro-3-nitrobenzaldehyde phenyl)-4-fluoro-4-methyltetrahydropyran, its yield is 77%, and the structural characterization data are: 1 H NMR (600MHz, CDCl 3 )δ7.89(d,J=1.7Hz,2H),7.49(dd,J=15.4,5.1Hz,4H),4.71(dd,J=11.7,2.2Hz,2H),4.07-4.02(m,2H ),3.94-3.88(m,2H),2.08(ddt,J=13.8,9.0,2.3Hz,2H),1.85-1.73(m,4H),1.60-1.52(m,2H),1.43(s,3H ),1.40(s,3H); 13 C NMR (151MHz, CDCl 3 )δ148.09(s), 143.25-143.11(m), 131.89(s), 125.87-125.74(m), 73.25(s), 44.00-43.87(m), 36.18(s).
Embodiment 3
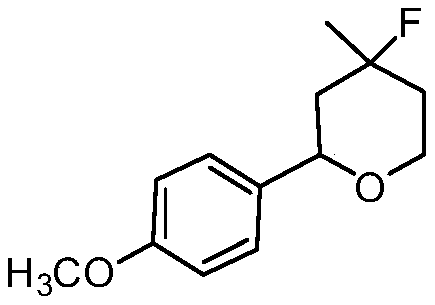
[0020] Synthesis of 4-fluoro-4-methyl-2-(4-methoxyphenyl)-tetrahydropyran with the following structural formula
[0021]
[0022] In this example, the p-nitrobenzaldehyde in Example 1 is replaced with equimolar 4-methoxybenzaldehyde, and other steps are the same as in Example 1 to obtain solid 4-fluoro-4-methyl-2-(4 -Methoxyphenyl)-tetrahydropyran, its yield is 68%, and structural characterization data is: 1 H NMR (600MHz, CDCl 3 )δ7.29(d,J=8.6Hz,4H),6.89(d,J=8.7Hz,4H),4.64(dd,J=11.7,1.9Hz,2H),4.05-4.00(m,2H), 3.97-3.90(m,2H),3.80(s,6H),2.05-1.99(m,2H),1.84-1.76(m,4H),1.73-1.65(m,2H),1.44(s,3H), 1.40(s,3H); 13 C NMR (151MHz, CDCl 3 )δ127.27(s), 92.80-92.67(m), 74.72(s), 64.02(s), 43.87(s), 36.61-36.48(m), 27.81(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com