Synthesis technology of cardiovascular medicine Apabetalone
A synthetic process and cardiovascular technology, applied in cardiovascular system diseases, drug combination, organic chemistry, etc., can solve the problems of inconvenient industrial production and achieve the effect of easy industrial production, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
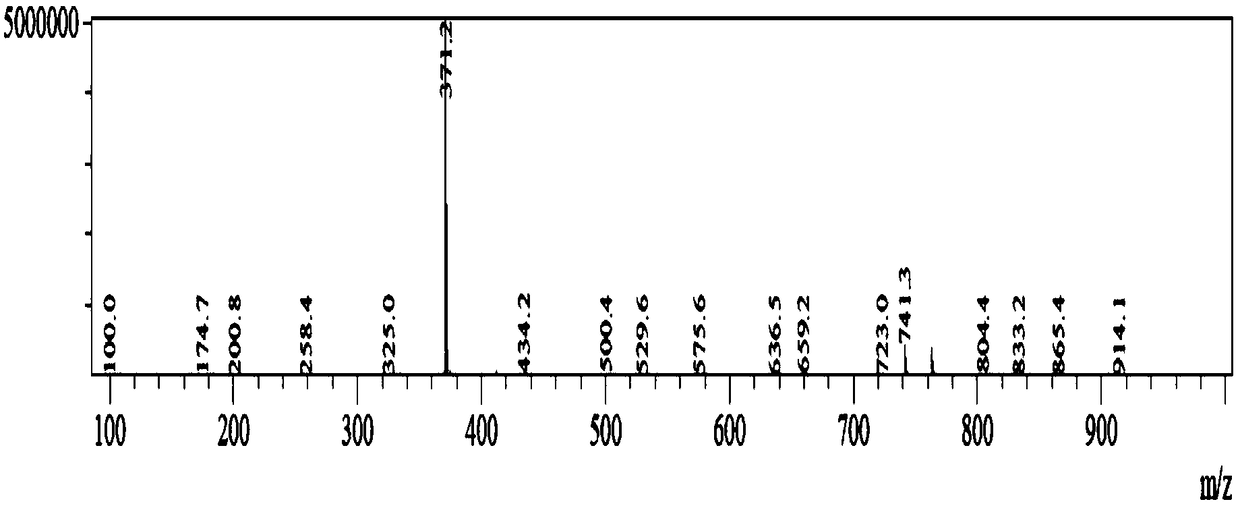
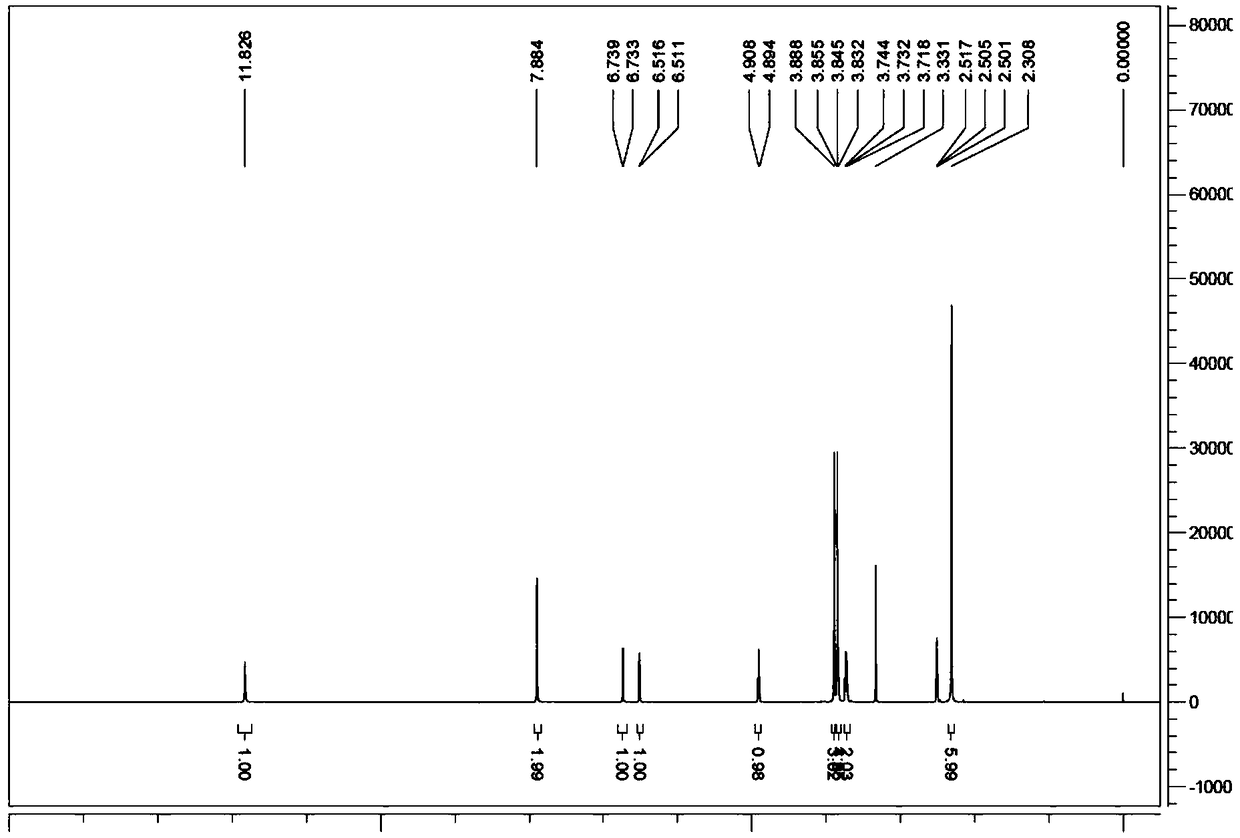
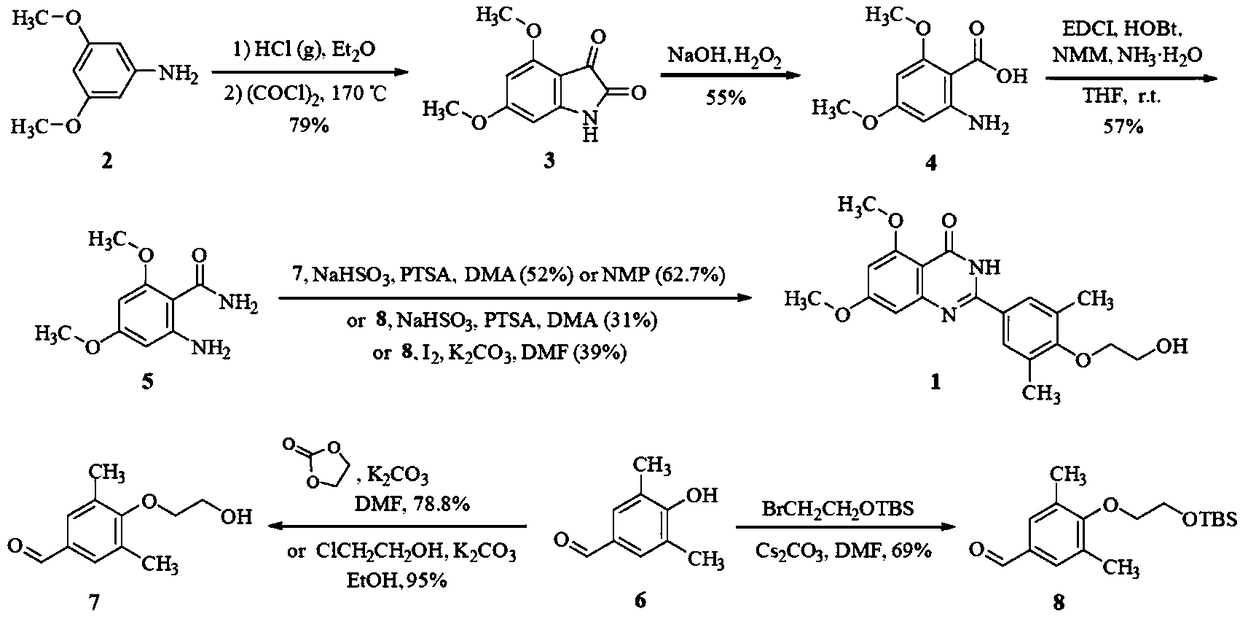
Image
Examples
Embodiment 1
[0029] Synthesis of N-(2,4-dimethoxyphenyl 1)-2-(oximino)acetamide:
[0030] Add 120g (0.73mol) of hydroxylamine sulfate, 90g (0.54mol) of chloral hydrate, and 200g (0.62mol) of sodium sulfate decahydrate into the reaction flask, stir thoroughly, and then add 2,4-dihydrochloride dissolved in 80ml hydrochloric acid and 60ml aqueous solution 81g (0.52mol) of methoxyaniline, heated and stirred to reflux for 2h, cooled and crystallized, suction filtered and dried to obtain 98.2g of N-(2,4-dimethoxyphenyl)-2-(oximino) Acetamide, yield 82.7%.
[0031] Synthesis of 5,6-dimethoxyisatin:
[0032]Add 400 g (4.08 mol) of concentrated sulfuric acid into the reaction flask, heat to 50°C, add 90 g (0.40 mol) of N-(2,4-dimethoxyphenyl)-2-(oximino)acetamide in batches, Control the internal temperature at 60-70°C. After adding, keep warm at 80°C for 30 minutes, pour into 10 times the reaction volume of water, let stand for 3 hours, crystallize, filter, wash with water until neutral, and recr...
Embodiment 2
[0038] Synthesis of N-(2,4-dimethoxyphenyl 1)-2-(oximino)acetamide:
[0039] Add 120g (0.73mol) of hydroxylamine sulfate, 90g (0.54mol) of chloral hydrate, and 200g (0.62mol) of sodium sulfate decahydrate into the reaction flask, stir thoroughly, and then add 2,4-dihydrochloride dissolved in 80ml hydrochloric acid and 60ml aqueous solution 81g (0.52mol) of methoxyaniline, heated and stirred to reflux for 2h, cooled and crystallized, suction filtered and dried to obtain 99.1g of N-(2,4-dimethoxyphenyl)-2-(oximino) Acetamide, yield 83.5%.
[0040] Synthesis of 5,6-dimethoxyisatin:
[0041] Add 400 g (4.08 mol) of concentrated sulfuric acid into the reaction flask, heat to 50°C, add 90 g (0.40 mol) of N-(2,4-dimethoxyphenyl)-2-(oximino)acetamide in batches, Control the internal temperature at 60-70°C, after adding, keep warm at 80°C for 30 minutes, pour into 10 times the reaction volume of water, let it stand for 3 hours, crystallize, filter, wash with water until neutral, and ...
Embodiment 3
[0047] Synthesis of N-(2,4-dimethoxyphenyl 1)-2-(oximino)acetamide:
[0048] Add 120g (0.73mol) of hydroxylamine sulfate, 90g (0.54mol) of chloral hydrate, and 200g (0.62mol) of sodium sulfate decahydrate into the reaction flask, stir thoroughly, and then add 2,4-dihydrochloride dissolved in 80ml hydrochloric acid and 60ml aqueous solution 81g (0.52mol) of methoxyaniline, heated and stirred to reflux for 2h, cooled and crystallized, suction filtered and dried to obtain 99.5g of N-(2,4-dimethoxyphenyl)-2-(oximino) Acetamide, yield 83.8%.
[0049] Synthesis of 5,6-dimethoxyisatin:
[0050] Add 400 g (4.08 mol) of concentrated sulfuric acid into the reaction flask, heat to 50°C, add 90 g (0.40 mol) of N-(2,4-dimethoxyphenyl)-2-(oximino)acetamide in batches, Control the internal temperature at 60-70°C, after adding, keep warm at 80°C for 30 minutes, pour into 10 times the reaction volume of water, let it stand for 3 hours, crystallize, filter, wash with water until neutral, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com