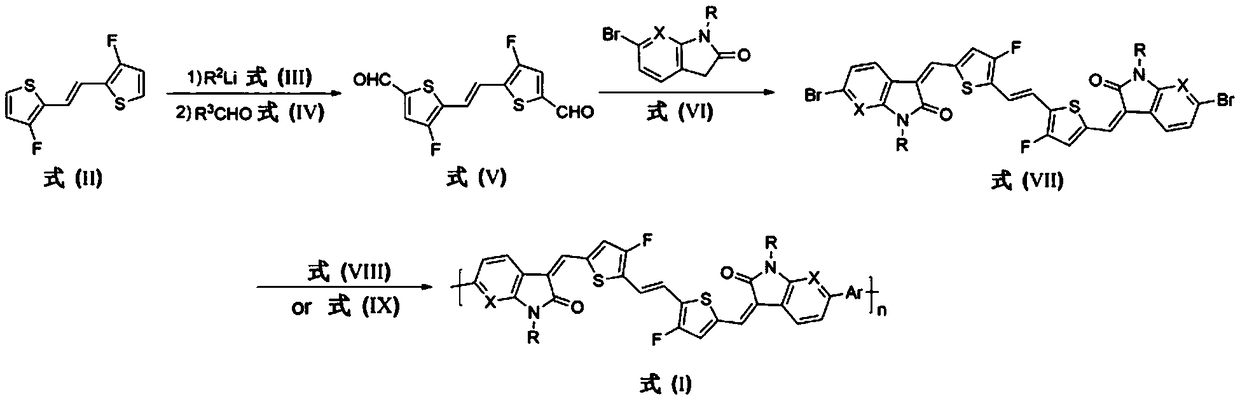
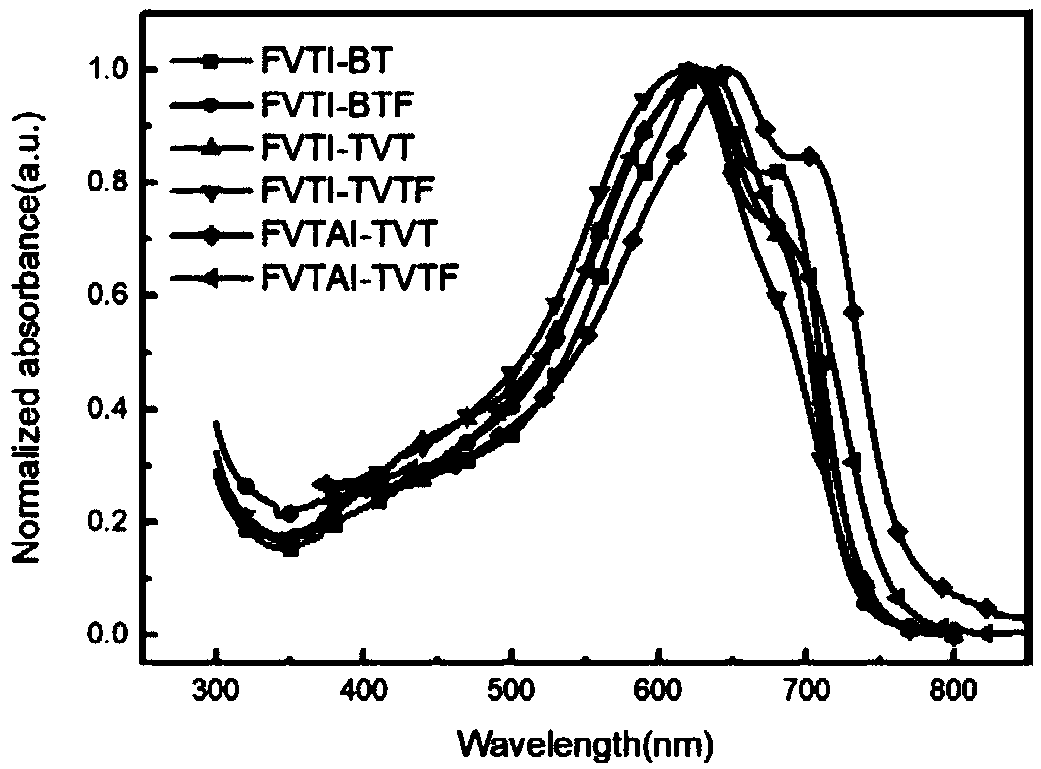
Polymer based on fluorodithiophene ethylene derivatives and application thereof in organic field-effect transistor
A technology of polymers and transistors, applied in the fields of organic chemistry, electric solid-state devices, semiconductor devices, etc., can solve problems such as unsatisfactory performance, and achieve a simple and easy synthesis route, a wide ultraviolet-visible light absorption spectrum, and good commercial application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
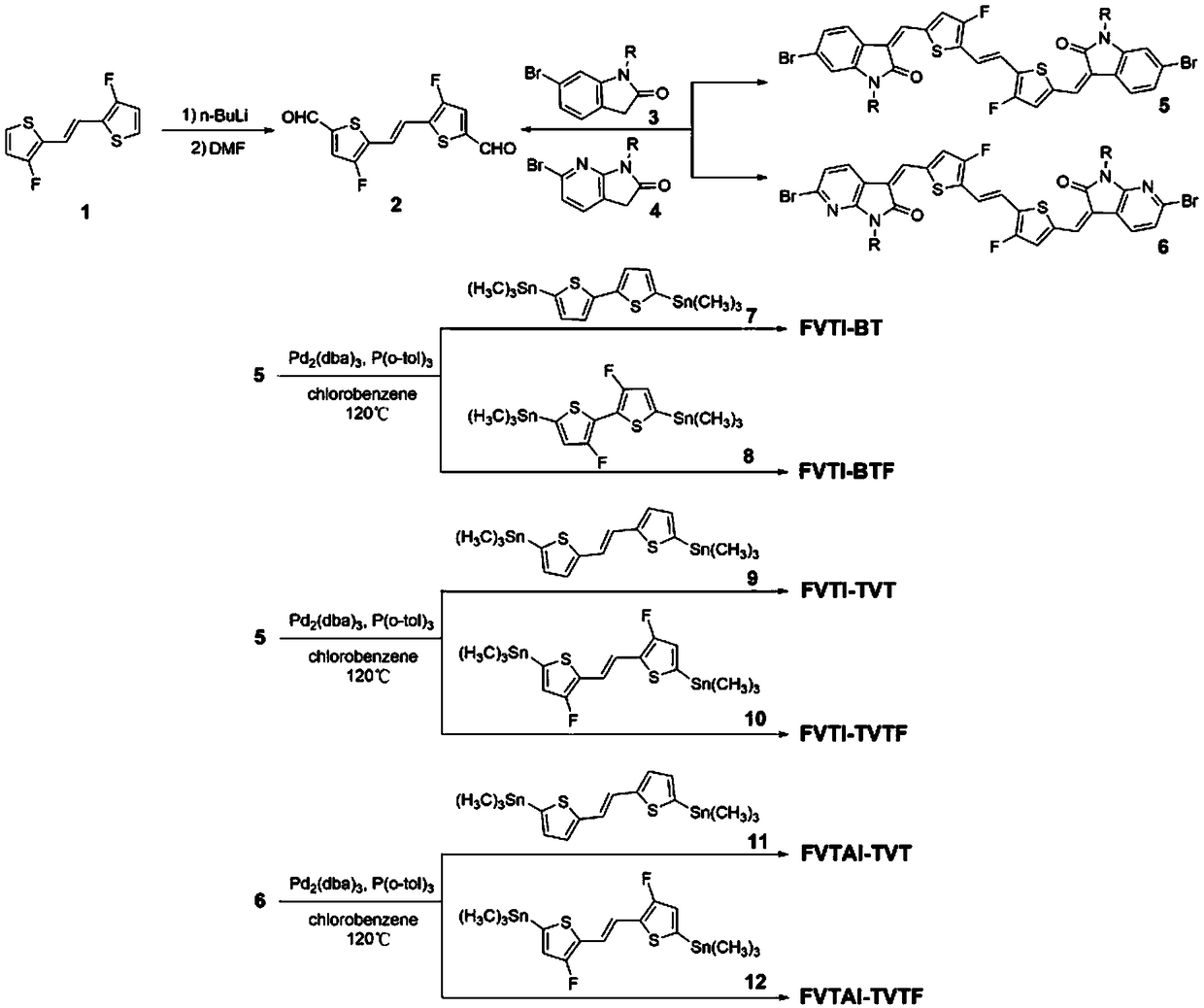
[0074] Embodiment 1, synthesis of polymer FVTI-BT (R=2-decyl dodecyl in formula I, X=C, Ar=2,2-dithiophene) (its synthetic route is as follows figure 2 shown)
[0075] 1) Synthesis of trans-1,2-bis(3-fluorothienyl)ethylene 1
[0076] To a solution of thiophene-2-carboxylic acid (17.0 g, 132.7 mmol) in anhydrous tetrahydrofuran (300 ml) was cooled to -78°C and slowly added 2.5M n-butyllithium in n-hexane (120 ml, 300 Millimoles). After completion, the reaction system was stirred at -78° C. for 30 minutes under the protection of nitrogen. Then N-fluorobisbenzenesulfonamide (50.0 g, 158.6 mmol) was dissolved in 300 ml of tetrahydrofuran and added to the reaction solution. After stirring at a low temperature of -78°C for 3 hours, hydrochloric acid was added to terminate the reaction, extracted with dichloromethane and washed with water several times, suspended in the solvent to obtain an intermediate product, and the amount of the intermediate product was accumulated by repeat...
Embodiment 2
[0103] Example 2, synthesis of polymer FVTI-BTF (R=2-decyl dodecyl in formula I, X=C, Ar=3,3-difluoro-2,2-dithiophene) (the synthesis route is as follows figure 2 shown)
[0104] The synthesis of compound 5 was carried out with reference to Example 1.
[0105] Compound 5 (265.5 mg, 0.20 mmol) and trimethyltin compound 8 (105.6 mg, 0.20 mmol), tris(dibenzylideneacetone) dipalladium (6.0 mg), tris(o-tolyl)phosphine ( 16.0 mg) and chlorobenzene (20 ml) were added into the reaction flask, and after deoxygenation at a low temperature of -78°C in nitrogen, the reaction was heated to 120°C under nitrogen protection for 15 minutes. After cooling, 200 ml of methanol / 6M HCl mixture (volume ratio 20:1) was added, stirred at room temperature for 2 hours, and filtered. The resulting solid was purified using a Soxhlet extractor. The extraction solvents were methanol, acetone, and n-hexane in sequence for 12 hours each, and then extracted with o-dichlorobenzene to obtain 258 mg of the ta...
Embodiment 3
[0109] Example 3, polymer FVTI-TVT synthesis (R=2-decyl dodecyl in formula I, X=C, Ar=trans-1,2-thienylethylene) (the synthesis route is as follows figure 2 shown)
[0110] The synthesis of compound 5 was carried out with reference to Example 1.
[0111] Compound 5 (200..0 mg 0.15 mmol) and trimethyltin compound 9 (77.7 mg, 0.15 mmol), tris(dibenzylideneacetone) dipalladium (6.0 mg), tris(o-tolyl) Phosphine (16.0 mg) and chlorobenzene (10 ml) were added to the reaction flask, and after deoxygenation at a low temperature of -78°C in nitrogen, the reaction was heated to 120°C under nitrogen protection for 15 minutes. After cooling, 200 ml of methanol / 6M HCl mixture (volume ratio 20:1) was added, stirred at room temperature for 2 hours, and filtered. The resulting solid was purified using a Soxhlet extractor. The extraction solvents were methanol, acetone, and n-hexane in sequence for 12 hours each, and then extracted with o-dichlorobenzene to obtain 196 mg of the target poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com