A kind of mercury ion detection reagent and preparation method and application thereof
A technology for detecting reagents and mercury ions, which is applied in the field of environmental chemistry, can solve the problems that the detection method cannot meet the actual needs, the requirements for the ability of the experimenters are high, and the cost of electrode production is high, so as to achieve industrial application, good selectivity, and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A mercury ion detection reagent, the detection reagent comprises nano gold particle solution and dithiothreitol solution.
[0047] In this embodiment, the initial concentration of the gold nanoparticle solution is 2.2nM; the initial concentration of the dithiothreitol solution is 20mM.
[0048] In this embodiment, the volume ratio of the dithiothreitol solution and the gold nanoparticle solution is 1:200.
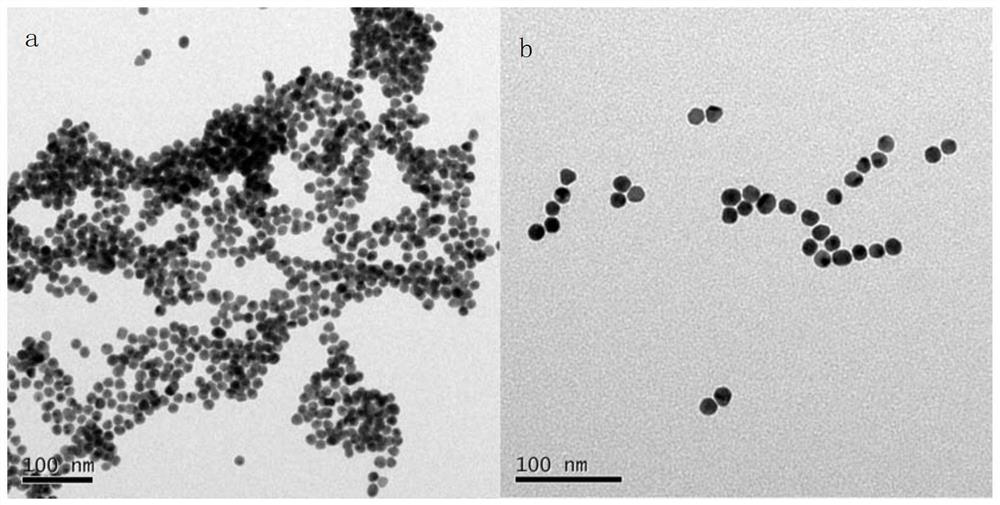
[0049] In this embodiment, the particle diameter of the gold nanoparticles in the gold nanoparticles solution is 13nm.
[0050] In this embodiment, the pH value of the detection reagent is 6.6.
[0051] A preparation method of the mercury ion detection reagent of the above-mentioned embodiment of the present invention, comprising the following steps:
[0052] (1) Preparation of nano-gold particle solution: take 91mL of chloroauric acid solution (solvent is water) with a concentration of 0.1g / L, heat it to boiling on an electric furnace and keep it in a boiling stat...
Embodiment 2
[0060] A preparation method for mercury ion detection reagent, comprising the following steps:
[0061] (1) Preparation of gold nanoparticles solution: same as in Example 1.
[0062] (2) Preparation of detection reagents:
[0063] Take 32 parts of the gold nanoparticle solution in step (1), each 2 mL, and divide them into two groups.
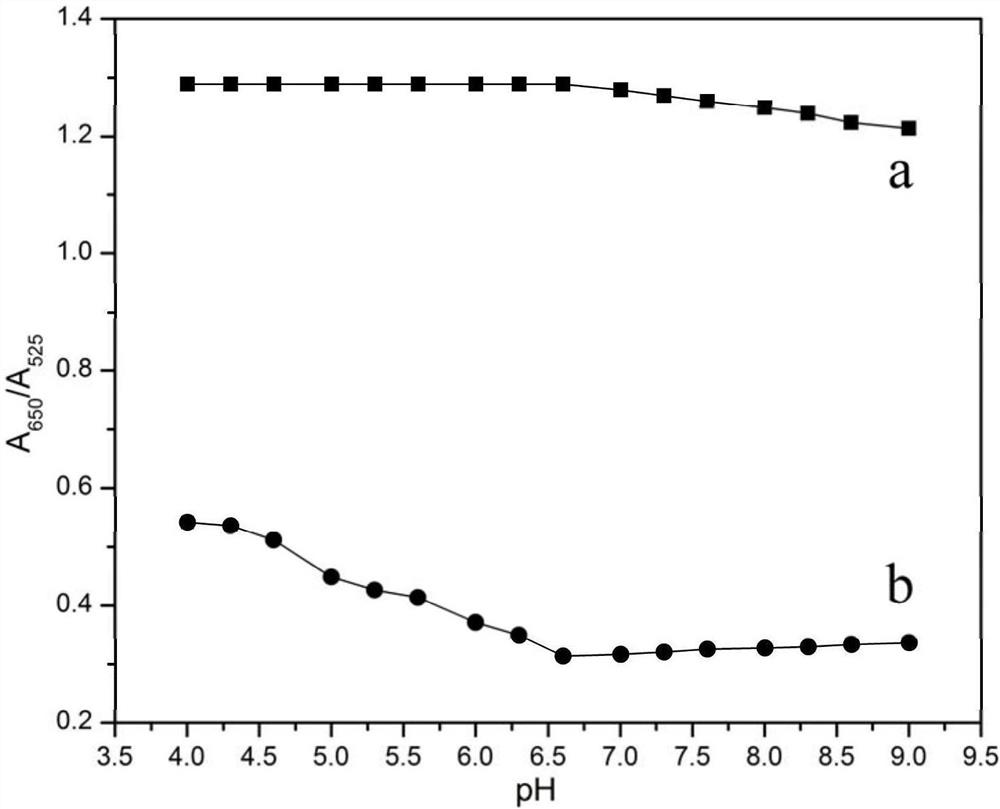
[0064] The first group: adding a volume of 10 μL and an initial molar concentration of DETL solution of 20 mM to each part of gold nanoparticle solution, standing for 2 hours under dark conditions, centrifuging and redissolving three times at a speed of 12000 r / min (centrifugal redissolving With a concentration of 10mM, pH is respectively 4.0, 4.3, 4.6, 5.0, 5.3, 5.6, 6.0, 6.3, 6.6, 7.0, 7.3, 7.6, 8.0, 8.3, 8.6, 9.0 phosphate buffer), each 15min, Detection reagents with pHs of 4.0, 4.3, 4.6, 5.0, 5.3, 5.6, 6.0, 6.3, 6.6, 7.0, 7.3, 7.6, 8.0, 8.3, 8.6, and 9.0 were obtained.
[0065] The second group: adding 10 μL of deionized water to each part ...
Embodiment 3
[0071] The application of a mercury ion detection reagent in detecting mercury ions comprises the following steps:
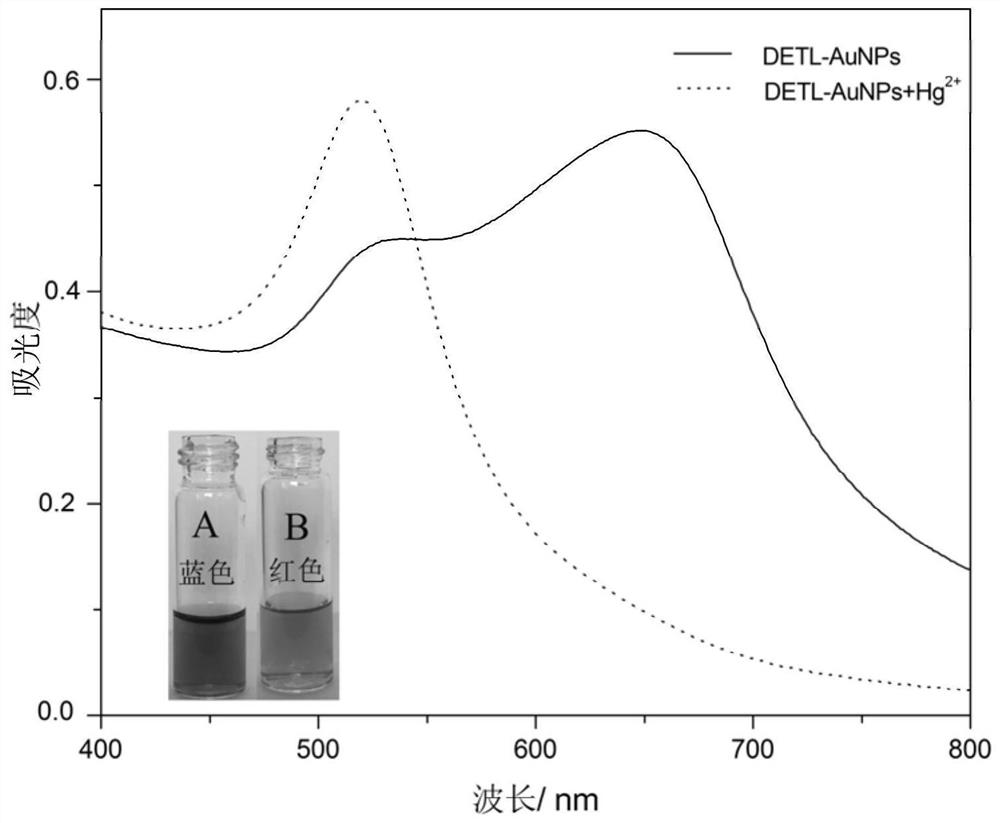
[0072] Take 10 parts of the mercury ion detection reagent prepared in Example 1, and add 30 μL of mercury ion solution with a concentration of 10 μM to each detection reagent to form a reaction system and start the reaction. The temperature during the reaction is 15 ° C, 18 °C, 20 °C, 24 °C, 28 °C, 30 °C, 32 °C, 34 °C, 38 °C and 40 °C, and the pH value of the system during the reaction was 6.6. After reacting for 20 minutes, detect the ultraviolet-visible absorption spectra of each group of systems, and compare the absorption values at 650nm and 525nm to obtain a ratio (A 650 / A 525 ), see the results Figure 4 .
[0073] Figure 4 It is a histogram of the ratio of absorbance at 650 nm to 525 nm corresponding to different reaction temperatures in Example 3 of the present invention. Such as Figure 4 As shown, with the increase of temperature, A 650 / A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com