Enzymatic synthesis method for L-selenium-methyl selenocysteine
A technology of methylselenocysteine and selenocysteine, applied in biochemical equipment and methods, enzymes, transferases, etc., can solve the problem of difficulty in large-scale actual production, complicated steps and harsh reaction conditions. and other problems, to achieve the effect of high practical promotion value, specificity and efficiency, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (continuous process)
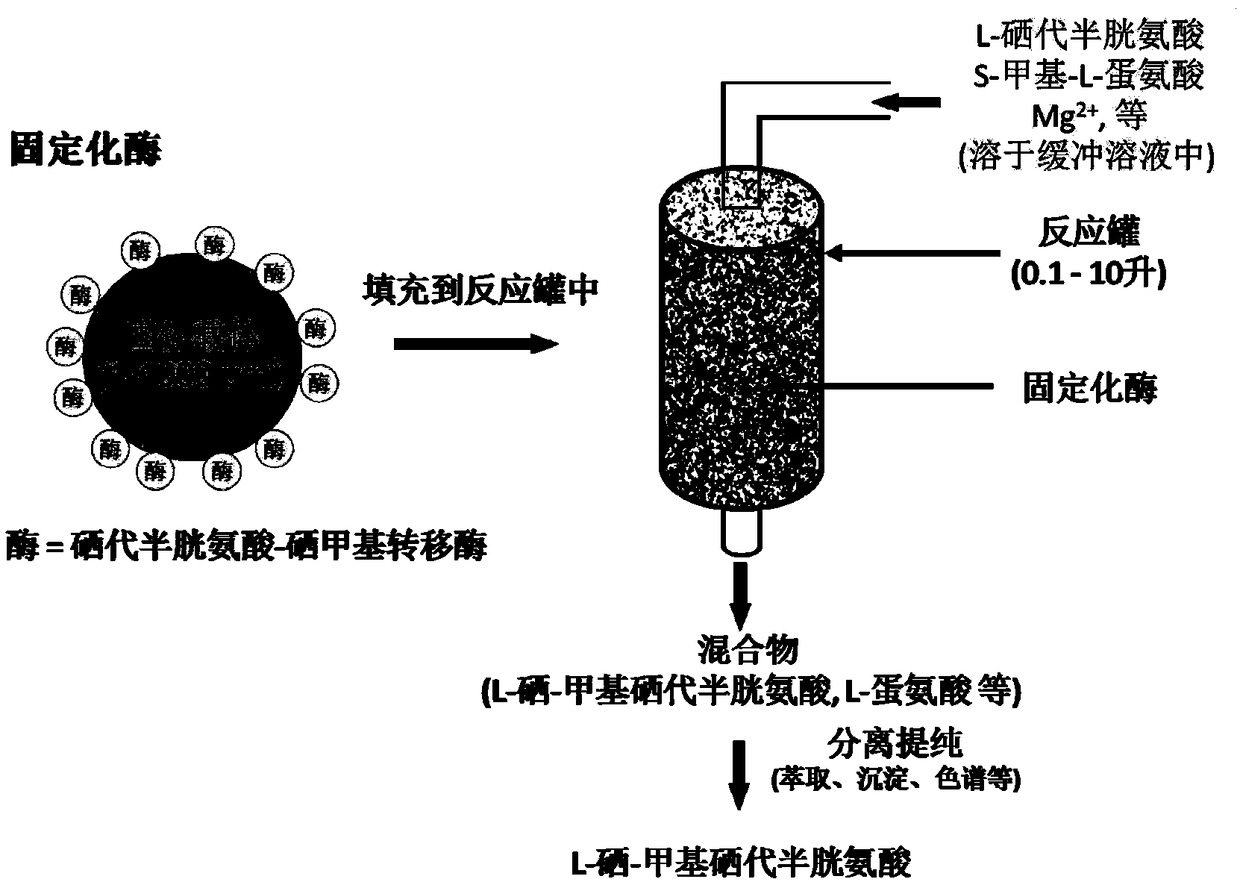
[0035] Step 1: 1.0 g of selenocysteine-selenomethyltransferase was immobilized on the surface of 100 g of polymer microparticles in advance, and packed into a 200 mL glass column.
[0036] Step 2: Take 200mL of water and pass nitrogen gas at room temperature for 30min to remove oxygen. Add L-selenocystine (3.34g, 10mmol) and β-mercaptoethanol (14mL, 200mmol), and stir with a magnet for 30min until L-selenocystine is completely dissolved and reduced to L-selenocysteine . During the process, the liquid level was kept under nitrogen.
[0037] Step 3: Take 0.8L ammonium acetate buffer solution (50mM, pH 6.0), add S-methyl-L-methionine (3.60g, 22mmol), magnesium acetate (0.3g, 2.1mmol), stir to dissolve, and then add step 2 The resulting L-selenocysteine solution.
[0038]Step 4: Keep the above solution (step 3) at 37°C and keep the liquid level under nitrogen, and pump it into the glass column (step 1) with the immobilized enzyme wi...
Embodiment 2
[0040] Embodiment 2 (continuous process)
[0041] Step 1: 0.5 g of selenocysteine-selenomethyltransferase was immobilized on the surface of 100 g of polyester fiber in advance, and packed into a 200 mL glass column.
[0042] Step 2: Take 200mL of water and pass nitrogen gas at room temperature for 30min to remove oxygen. Add L-selenocystine (1.67g, 5mmol) and sodium borohydride (3.78g, 100mmol), and stir with a magnet for 30min until L-selenocystine is completely dissolved and reduced to L-selenocysteine . During the process, the liquid level was kept under nitrogen.
[0043] Step 3: Take 0.8L ammonium acetate buffer solution (20mM, pH 7.0), add S-methyl-L-methionine (1.80g, 11mmol), magnesium acetate (0.3g, 2.1mmol), stir to dissolve, and then add step 2 The resulting L-selenocysteine solution.
[0044] Step 4: Keep the above solution (step 3) at 30° C. with the liquid level under nitrogen, and pump it into the glass column (step 1) with the immobilized enzyme with a sm...
Embodiment 3
[0046] Embodiment 3 (batch process)
[0047] Step 1: Immobilize 0.25 g of selenocysteine-selenomethyltransferase on the surface of 50 g of polymer microparticles in advance, and set aside.
[0048] Step 2: Take 200mL of water and pass nitrogen gas at room temperature for 30min to remove oxygen. Add L-selenocystine (1.67g, 5mmol) and β-mercaptoethanol (7.0mL, 100mmol), and stir with a magnet for 30min until L-selenocystine is completely dissolved and reduced to L-selenocysteine acid. During the process, the liquid level was kept under nitrogen.
[0049] Step 3: Take 0.8L of ammonium acetate buffer solution (25mM, pH 6.0), add S-methyl-L-methionine (1.80g, 11mmol), magnesium acetate (0.3g, 2.1mmol), and stir with a magnet to dissolve.
[0050] Step 4: Mix immobilized enzyme (step 1), L-selenocysteine solution (step 2) and S-methyl-L-methionine buffer solution (step 3). The liquid level was maintained under nitrogen at 37°C with slow agitation using a magnet. After 16 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com