Synthetic method of sitagliptin phosphate impurities
A technology of sitagliptin phosphate and a synthesis method, which is applied in the field of organic synthesis and achieves the effects of high content, low requirements for reaction equipment and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
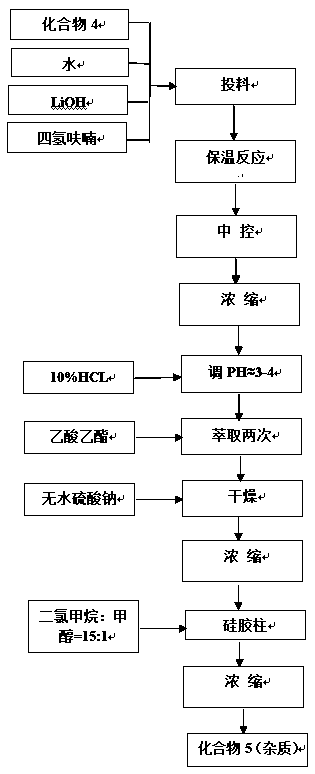
Image
Examples
Embodiment 1
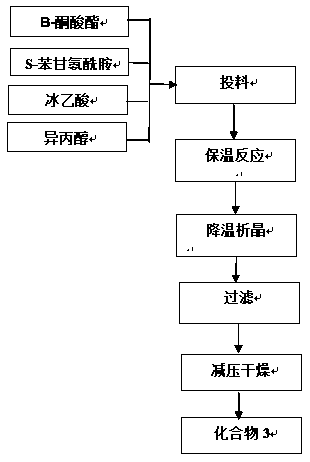
[0035] Step 1. Schiff base reaction
[0036] Add β-ketoester (3.5g), S-benzylglycineamide (2.14g), and glacial acetic acid (4.5g) into isopropanol (50ml), under nitrogen protection, heat to 40°C and keep it warm for 16h. Solids began to precipitate after cooling down to 5-10°C. After 30 minutes, suction filtered and washed the filter cake with an appropriate amount of isopropanol. The filter cake was dried under reduced pressure at 40°C to obtain solid compound 3: (S,Z)-methyl 3-( 2-Amino-2-oxo-1-phenylethylamino)-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester 4.5g, purity 90%, yield 76% . The NMR structure identification results are as follows:
[0037] 1HNMR (400MHz, DMSO, D2O),
[0038] δ: 3.32(d, J=16.4Hz, 1H), 3.47(d, J=16.7Hz, 1H), 4.20(s, 1H), 5.16(d.J=7.9Hz, 1H), 7.15(dd, J=17.5 , 9.2Hz, 1H), 7.37-7.22 (m, 6H), 7.50-7.39 (m, 1H), 7.73 (s, 1H), 9.53 (d, J = 7.7Hz, 1H).
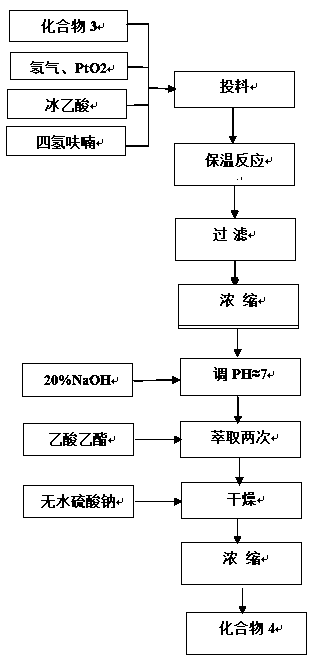
[0039] Step 2, hydrogenation reaction
[0040] Then compound 3 (4.0g), PtO2 (0.4g), gl...
Embodiment 2
[0046] Step 1. Schiff base reaction
[0047] Add β-ketoester (35g), S-benzylglycine amide (21.4g) and glacial acetic acid (45g) into ethanol (500ml), under nitrogen protection, heat to 40°C for 16h and then cool down for 5-10 After 30 minutes, it was filtered with suction, and the filter cake was washed with an appropriate amount of ethanol. The filter cake was dried under reduced pressure at 40°C to obtain the solid compound 3 ((S, Z)-methyl 3-(2-amino-2- Oxo-1-phenylethylamino)-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester) 47g, purity 92%, yield 79%. The NMR structure identification results are as follows:
[0048] 1HNMR (400MHz, DMSO, D2O),
[0049] δ: 3.32(d, J=16.4Hz, 1H), 3.47(d, J=16.7Hz, 1H), 4.20(s, 1H), 5.16(d.J=7.9Hz, 1H), 7.15(dd, J=17.5 , 9.2Hz, 1H), 7.37-7.22 (m, 6H), 7.50-7.39 (m, 1H), 7.73 (s, 1H), 9.53 (d, J = 7.7Hz, 1H).
[0050] Step 2, hydrogenation reaction
[0051] Then compound 3 (40g), PtO2 (4g), glacial acetic acid (45ml) and ethanol (12...
Embodiment 3
[0057] Step 1. Schiff base reaction
[0058] Add β-ketoester (350g), S-benzylglycineamide (214g) and glacial acetic acid (450g) into ethanol (5000ml), under nitrogen protection, heat to 40°C for 16 hours, then cool down by 5-10°C After 30 minutes, a solid was precipitated, and after 30 minutes, it was suction filtered, and the filter cake was washed with an appropriate amount of ethanol, and the filter cake was dried under reduced pressure at 40°C to obtain a solid compound 3 ((S, Z)-methyl 3-(2-amino-2-oxo Substituent-1-phenethylamino)-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester) 480g, purity 92%, yield 80%. The NMR structure identification results are as follows:
[0059] 1HNMR (400MHz, DMSO, D2O),
[0060] δ: 3.32(d, J=16.4Hz, 1H), 3.47(d, J=16.7Hz, 1H), 4.20(s, 1H), 5.16(d.J=7.9Hz, 1H), 7.15(dd, J=17.5 , 9.2Hz, 1H), 7.37-7.22 (m, 6H), 7.50-7.39 (m, 1H), 7.73 (s, 1H), 9.53 (d, J = 7.7Hz, 1H).
[0061] Step 2, hydrogenation reaction
[0062] Then compound 3 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com