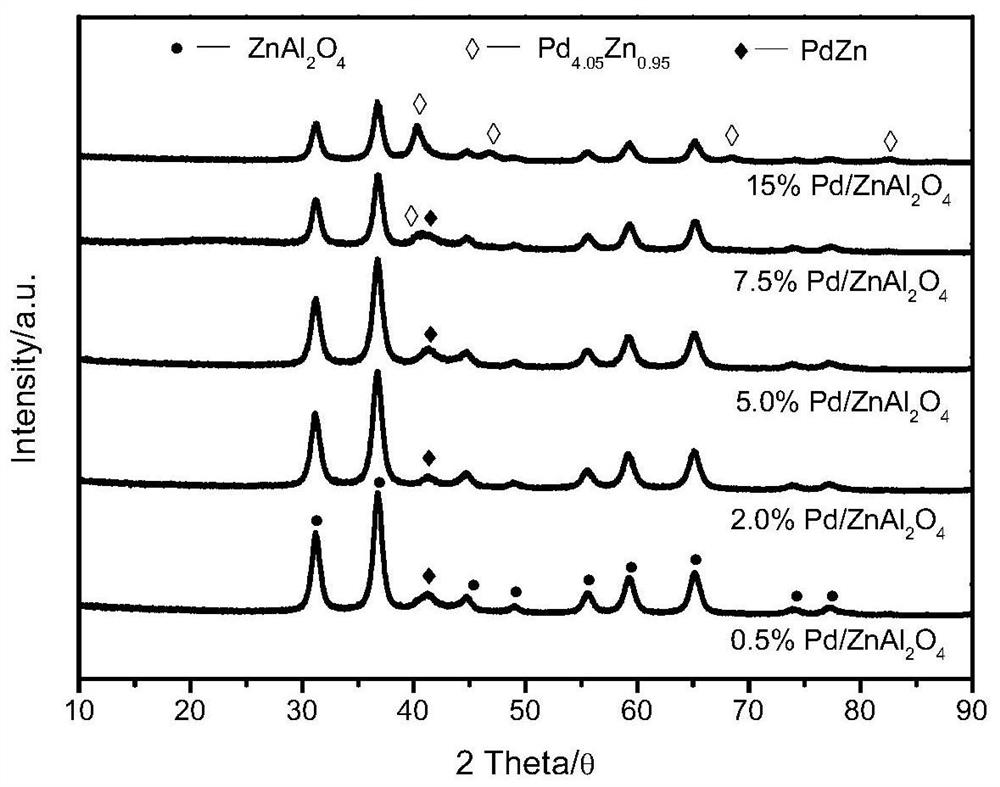
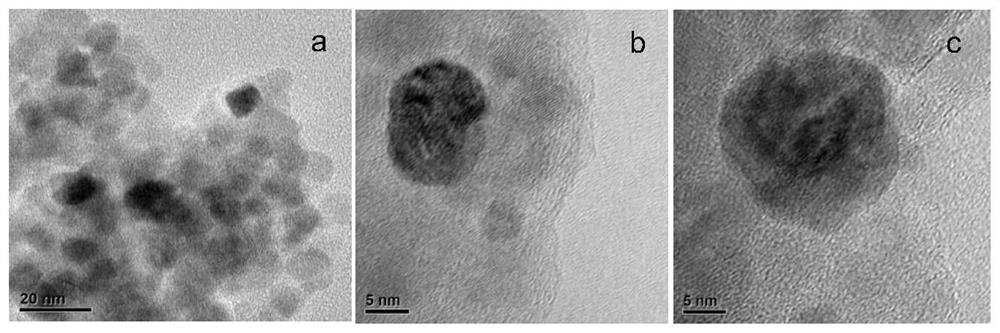
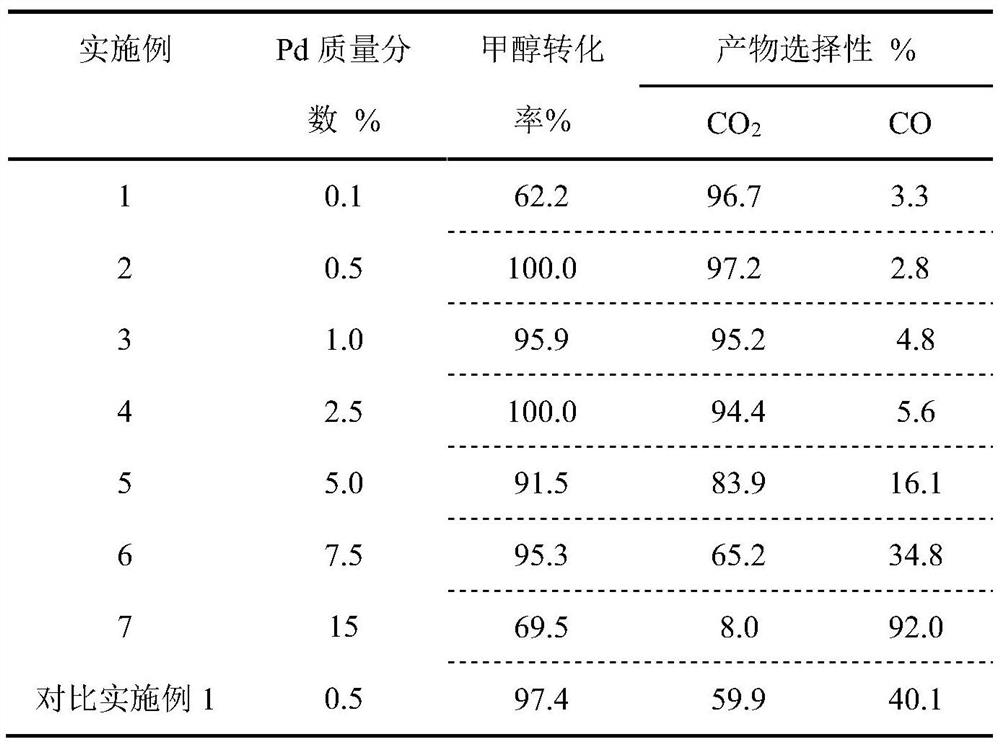
One will pd/znal 2 o 4 Catalyst used in methanol steam reforming hydrogen production method
A technology for reforming hydrogen and methanol steam, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve copper-based catalyst coking and catalyst sintering deactivation , prone to spontaneous combustion and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Pd / ZnAl with a mass fraction of Pd of 0.1% 2 o 4 The specific method of hydrogen production by catalytic methanol steam reforming with catalyst is as follows:
[0032] 1) Take 1.25mL of Pd(NO 3 ) 2 2H 2 O solution (2mg / mL) in a beaker, then 1g ZnAl 2 o 4 The carrier was added to the solution, stirred at room temperature for 10 h, then transferred to a water bath at 80 °C to continue stirring, the solvent was evaporated to dryness, and the obtained dried sample was placed in an oven at 120 °C to dry overnight, and finally calcined at 400 °C for 2 h to obtain Pd. Pd / ZnAl with a mass fraction of 0.1% 2 o 4 catalyst.
[0033] 2) The pretreatment conditions before the use of the catalyst are: the reducing atmosphere is H 2 , the reduction temperature is 350°C, and the reduction time is 1h
[0034] 3) The evaluation conditions of the catalyst are as follows: the reaction pressure is 0.1MPa, the reaction temperature is 150°C, and the mass space velocity of the liquid...
Embodiment 2
[0036] Pd / ZnAl with a mass fraction of Pd of 0.5% 2 o 4 The specific method of the catalyst catalytic methanol steam reforming hydrogen production is as follows:
[0037] 1) Take 6.25mL of Pd(NO 3 ) 2 2H 2 O solution (2mg / mL) in a beaker, then 1g ZnAl 2 o 4 The carrier was added to the solution, stirred at room temperature for 10 hours, then transferred to a water bath at 80°C to continue stirring, the solvent was evaporated to dryness, and the obtained dried sample was placed in an oven at 120°C to dry overnight, and finally calcined at 400°C for 3 hours to obtain the mass of Pd Fraction 0.5% Pd / ZnAl 2 o 4 catalyst.
[0038] 2) The pretreatment conditions before the use of the catalyst are: the reducing atmosphere is H 2 , the reduction temperature is 350°C, and the reduction time is 1h
[0039] 3) The evaluation conditions of the catalyst are as follows: the reaction pressure is 0.5MPa, the reaction temperature is 250°C, and the mass space velocity of the liquid ra...
Embodiment 3
[0041] Pd / ZnAl with a mass fraction of Pd of 1.0% 2 o 4 The specific method of the catalyst catalytic methanol steam reforming hydrogen production is as follows:
[0042] 1) Take 12.5mL of Pd(NO 3 ) 2 2H 2 O solution (2mg / mL) in a beaker, then 1g ZnAl 2 o 4 The carrier was added to the solution, stirred at room temperature for 10 hours, then transferred to a water bath at 80°C to continue stirring, and the solvent was evaporated to dryness. The dried sample obtained was placed in an oven at 120°C to dry overnight, and finally calcined at 400°C for 4 hours to obtain the mass of Pd. Fraction 1.0% Pd / ZnAl 2 o 4 catalyst.
[0043] 2) The pretreatment conditions before the use of the catalyst are: the reducing atmosphere is H 2 , the reduction temperature is 350°C, and the reduction time is 1h
[0044] 3) The evaluation conditions of the catalyst are as follows: the reaction pressure is 1.0MPa, the reaction temperature is 300°C, and the mass space velocity of the liquid r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com