Compound for degrading FAK (Focal Adhesion Kinase) protein in targeting manner and application thereof
A compound, hydrate technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
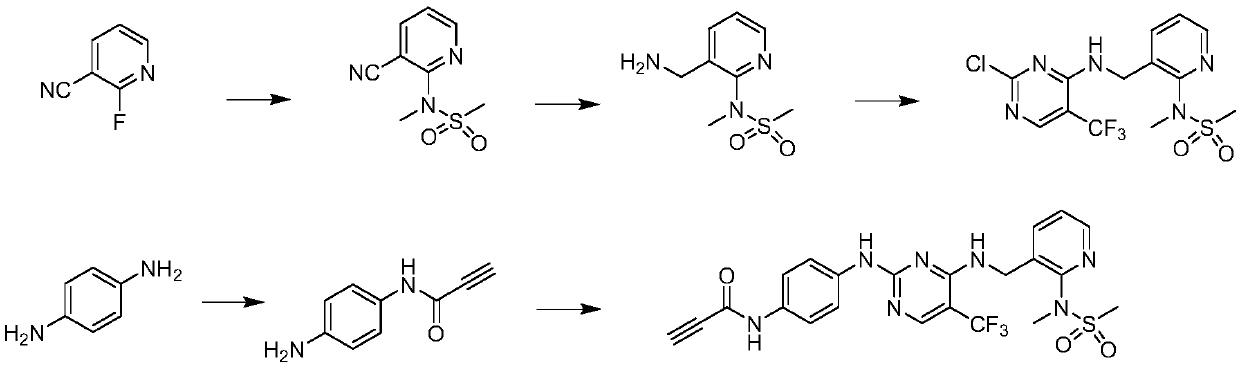
[0190] (1) Preparation of Intermediate 1a
[0191]
[0192] Add 2 g of p-phenylenediamine, 954 μl of propiolic acid, and 15 ml of chloroform into a 250 ml round-bottom flask, add anhydrous DMF until completely dissolved, cool to zero, and add 3.18 g of DCC in 20 ml of chloroform. Stir at zero for 1 hour, filter to obtain a clear filtrate, and then use DCM:MeOH=20:1 to pass through a silica gel column to obtain intermediate 1a. 1 H-NMR (400MHz, DMSO-d 6 ,ppm):10.35(s,1H),7.24(d,J=8.60Hz,2H),6.50(d,J=8.64Hz,2H),4.97(s,2H),4.25(s,1H).LC -MS: calculated for C 9 h 9 N 2 O[M+H] + :161.06, found 161.28.
[0193] (2) Preparation of Intermediate 2a
[0194]
[0195]Add 2 grams of N-methylmethanesulfonamide, 30 milliliters of anhydrous DMF and 2.1 grams of potassium tert-butoxide into a 100 milliliter round bottom flask, stir at room temperature for 20 minutes, then add 2.2 grams of 2-fluoro-3-cyanopyridine, The reaction was refluxed for 3 hours, quenched with water, extra...
Embodiment 1
[0205] The preparation of compound shown in embodiment 1 formula 1~38
[0206]
[0207] Add 17 mg of Pomalidomide terminal derivatives, 20 mg of intermediate 5a, 7 mg of CuSO in a 5 ml round bottom flask 4 , 23mg sodium ascorbate, 0.1ml water and 0.8ml tert-butanol. After stirring at 70°C for 6 hours, it was washed with water and extracted with DCM. The compound represented by formula 1 was obtained by passing through a silica gel column with dichloromethane:methanol=30:1, and the yield was 61%.
[0208] 1 H-NMR (400MHz, CDCl 3 ,ppm):8.88(s,1H),8.43(dd,J=1.80Hz,J=4.68Hz,1H),8.27(s,1H),8.18(s,1H),7.81(d,J=7.84Hz ,1H),7.59-7.46(m,6H),7.25(m,1H),7.09(d,J=7.04Hz,2H),6.86(d,J=8.52Hz,1H),6.46(t,J= 5.28Hz, 1H), 6.04(s, 1H), 4.95-4.88(m, 3H), 4.65(q, J=4.52Hz, 2H), 3.97(t, J=4.68Hz, 2H), 3.71(t, J=5.12Hz, 2H), 3.46(q, J=5.48Hz, 2H), 3.28(s, 3H), 3.07(s, 3H), 2.76-2.60(m, 3H), 2.10-2.00(m, 1H ).LC-MS: calculated for C 39 h 39 f 3 N 13 o 8 S[M+H] + :906.26, found 906.88....
Embodiment 2
[0228] Embodiment 2 The degradative activity of compound of the present invention to FAK
[0229]The compound of the present invention has a strong degradation activity to FAK, and the small molecular compound represented by formulas 1-10 is taken as an example to test below.
[0230] Cell seeding and small molecule compound treatment:
[0231] Digest PA1 cells with 90% cell confluency with 0.25% trypsin at 37°C for 1 minute, add Mycos'5A medium containing 10% FBS to stop the digestion and pipette into a single cell suspension, 15 ml centrifuge tube Collect single cells, centrifuge at 800rpm for 3 minutes, resuspend the cells with fresh medium, and count the number of cells on a cell counting plate. Cells were seeded in 24-well plates at a cell number of 800k per well.
[0232] After 12-24 hours, add the small molecular compound to be tested (1000x) for treatment, and 2 Incubate for 8 hours in the incubator. After 8 hours of compound treatment, cells were harvested and pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com