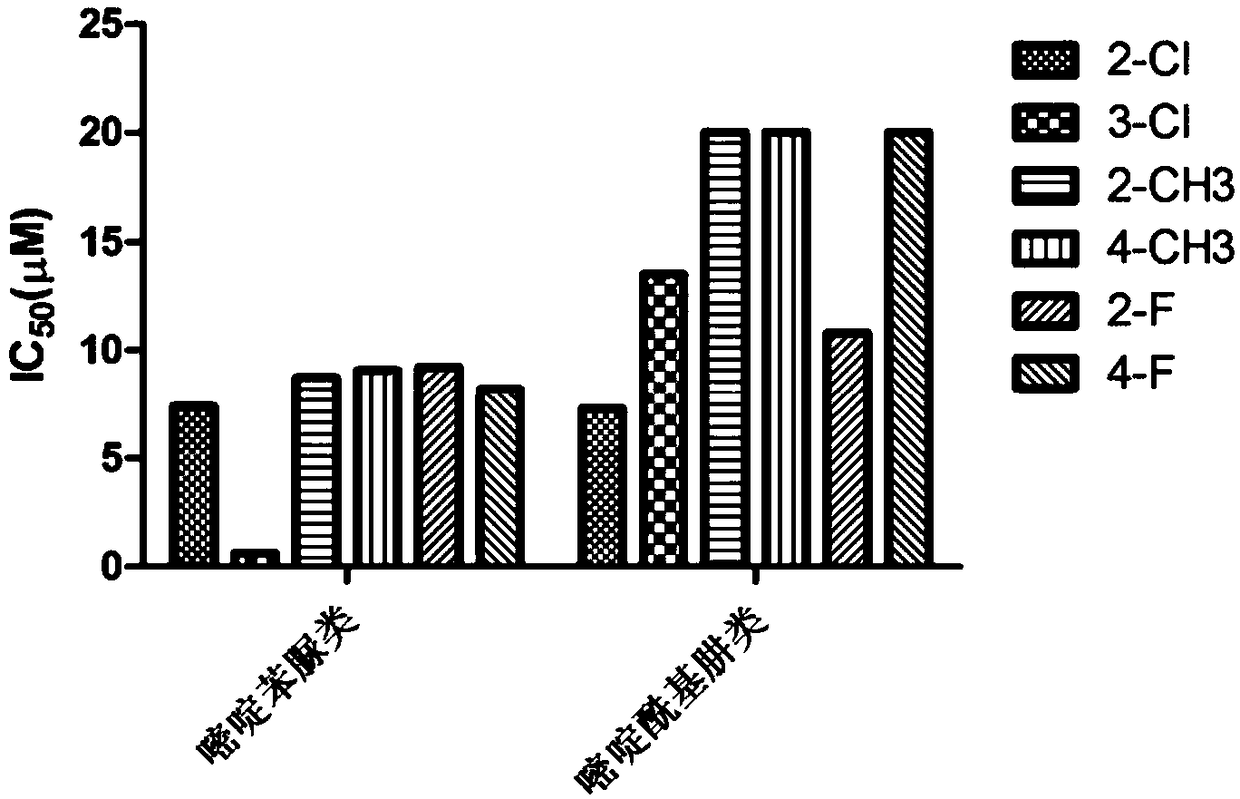
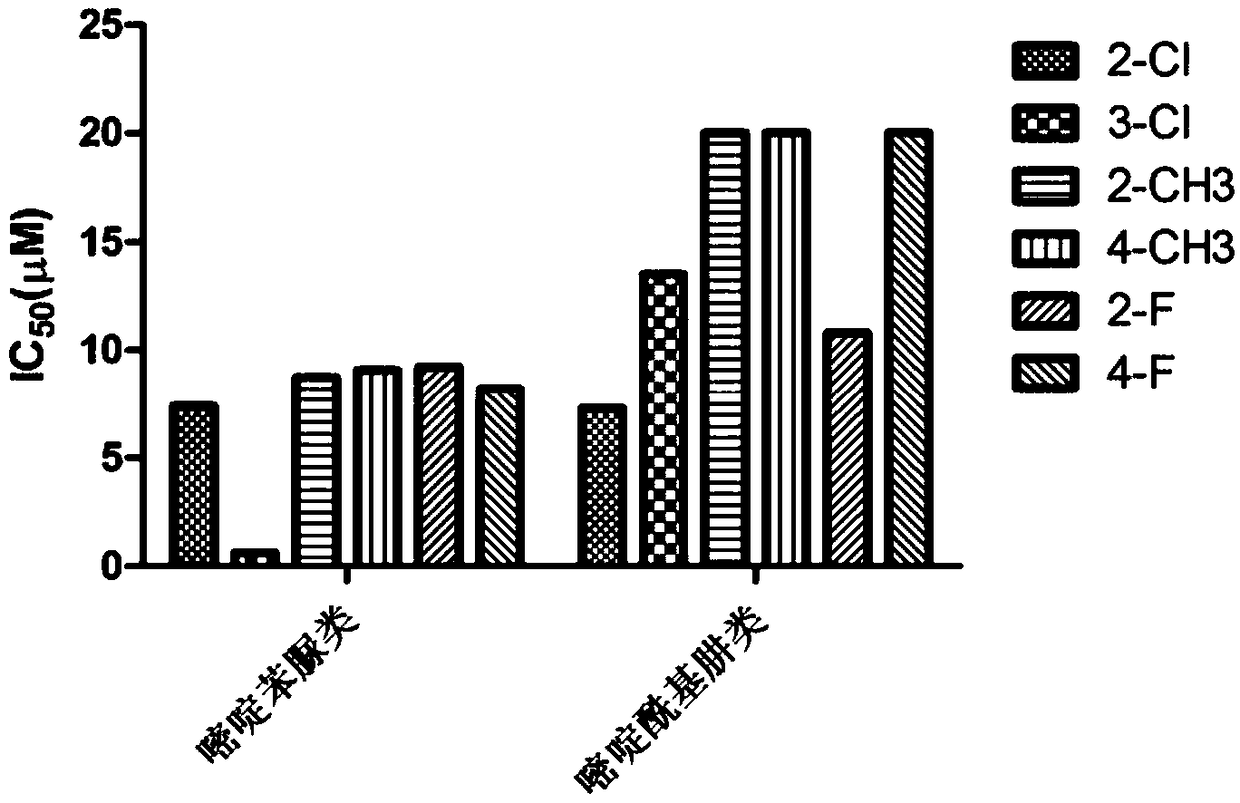
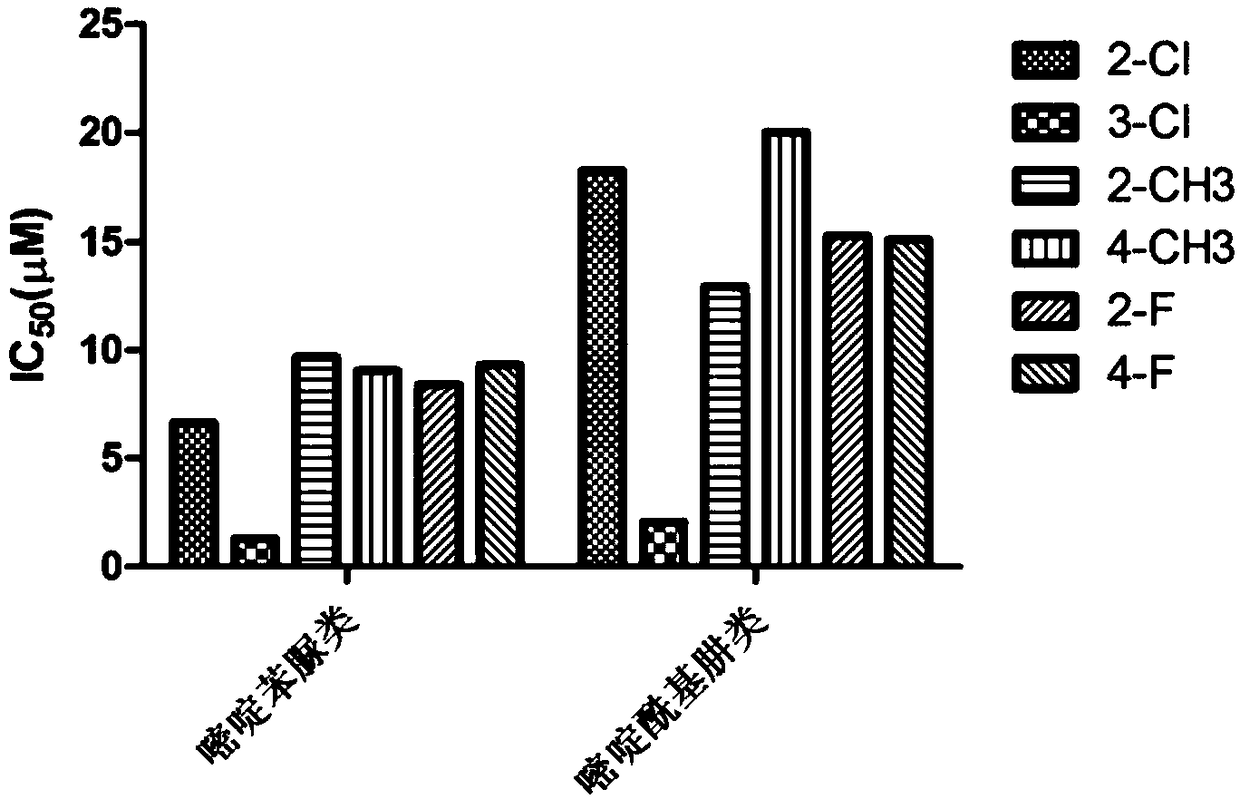
Pyrimidine phenylurea anti-tumor compound and preparation method and application thereof
A pyrimidine phenylurea and anti-tumor technology, which is applied in the field of medicine, can solve problems such as unseen reports, achieve the effects of reducing the possibility of drug resistance, simplifying the synthesis steps, and improving the anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of N'-(2-methylphenyl)-3-(uracil-5-sulfonamido)phenylurea A1.
[0055] a. Preparation of uracil-5-sulfonyl chloride: at 25-40°C, add uracil (5g, 44.63mmoL) and thionyl chloride (15ml, 89.26mmoL) into a 100mL reaction flask, activate 1.5-3 Add chlorosulfonic acid (25g, 133.89mmoL) to react at 50-65°C for 4-5h, and continue to react at 70-90°C for 5-8h; add the reaction solution into ice water, precipitate out, suction filter, wash with water for 50 C and dried to obtain 4.50 g of white solid, yield: 70%.
[0056] b. Dissolve 3-nitrobenzohydrazide (5.00g, 27.60mmol) in dichloromethane in a 100mL reaction flask at 0°C, and then add sodium nitrite (3.81g, 55.20mmol) aqueous solution Diluted hydrochloric acid (2.01g, 55.20mmol) was added dropwise, reacted for about 1-2 hours, separated and dried at 50°C to obtain 3-nitrobenzoyl azide yellow solid (5.00g, 26.05mmol), yield 94.28%.
[0057] c.: Dissolve 3-nitrobenzoyl azide (5.00g, 26.05mmol) in carbon tetrachlor...
Embodiment 2
[0062] Preparation of N'-(3-methylphenyl)-3-(uracil-5-sulfonamido)phenylurea A2
[0063] a. Preparation of uracil-5-sulfonyl chloride: at 25-40°C, add uracil (5g, 44.63mmoL) and thionyl chloride (15ml, 89.26mmoL) into a 100mL reaction flask, activate 1.5-3 Add chlorosulfonic acid (25g, 133.89mmoL) to react at 50-65°C for 4-5h, and continue to react at 70-90°C for 5-8h; add the reaction solution into ice water, precipitate out, suction filter, wash with water for 50 C and dried to obtain 4.50 g of white solid, yield: 70%.
[0064] b. Dissolve 3-nitrobenzohydrazide (5.00g, 27.60mmol) in dichloromethane in a 100mL reaction flask at 0°C, and then add sodium nitrite (3.81g, 55.20mmol) aqueous solution Diluted hydrochloric acid (2.01g, 55.20mmol) was added dropwise, reacted for about 1-2 hours, separated and dried at 50°C to obtain 3-nitrobenzoyl azide yellow solid (5.00g, 26.05mmol), yield 94.28%.
[0065] c.: Dissolve 3-nitrobenzoyl azide (5.00g, 26.05mmol) in carbon tetrachlori...
Embodiment 3
[0070] N'-(4-tolyl)-3-(uracil-5-sulfonamido)phenylurea A3 was prepared as in Example 1
[0071] 1 H NMR (600MHz, DMSO-d 6 )δ11.81(br.s.,1H),11.62(br.s.,1H),10.13(br.s.,1H),8.70(br.s.,1H),8.52(br.s., 1H),8.05(br.s.,1H),7.57-7.22(m,3H),7.20-6.91(m,4H),6.70(d,J=7.3Hz,1H),2.24(s,3H)MS (ESI,m / z):414.40[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com