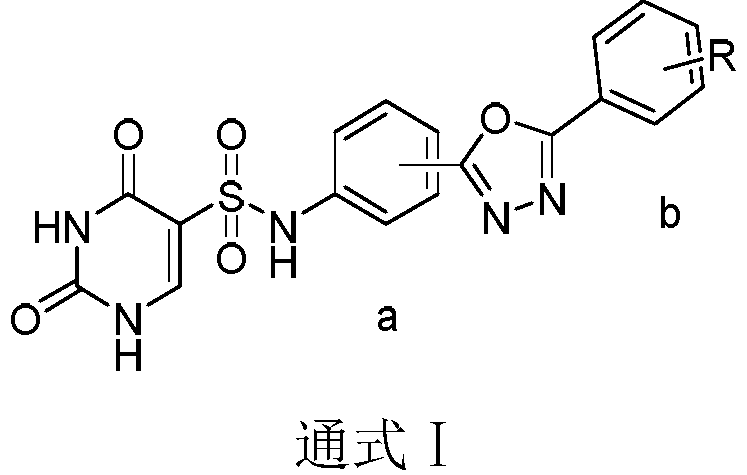
Pyrimidine antitumor compound with 1,3,4-oxadiazole structure and its preparation method and application
An oxadiazole pyrimidine and anti-tumor drug technology, applied in the field of medicine, can solve unseen problems, and achieve the effects of simplifying synthesis steps, improving anti-tumor effect, and excellent anti-proliferation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
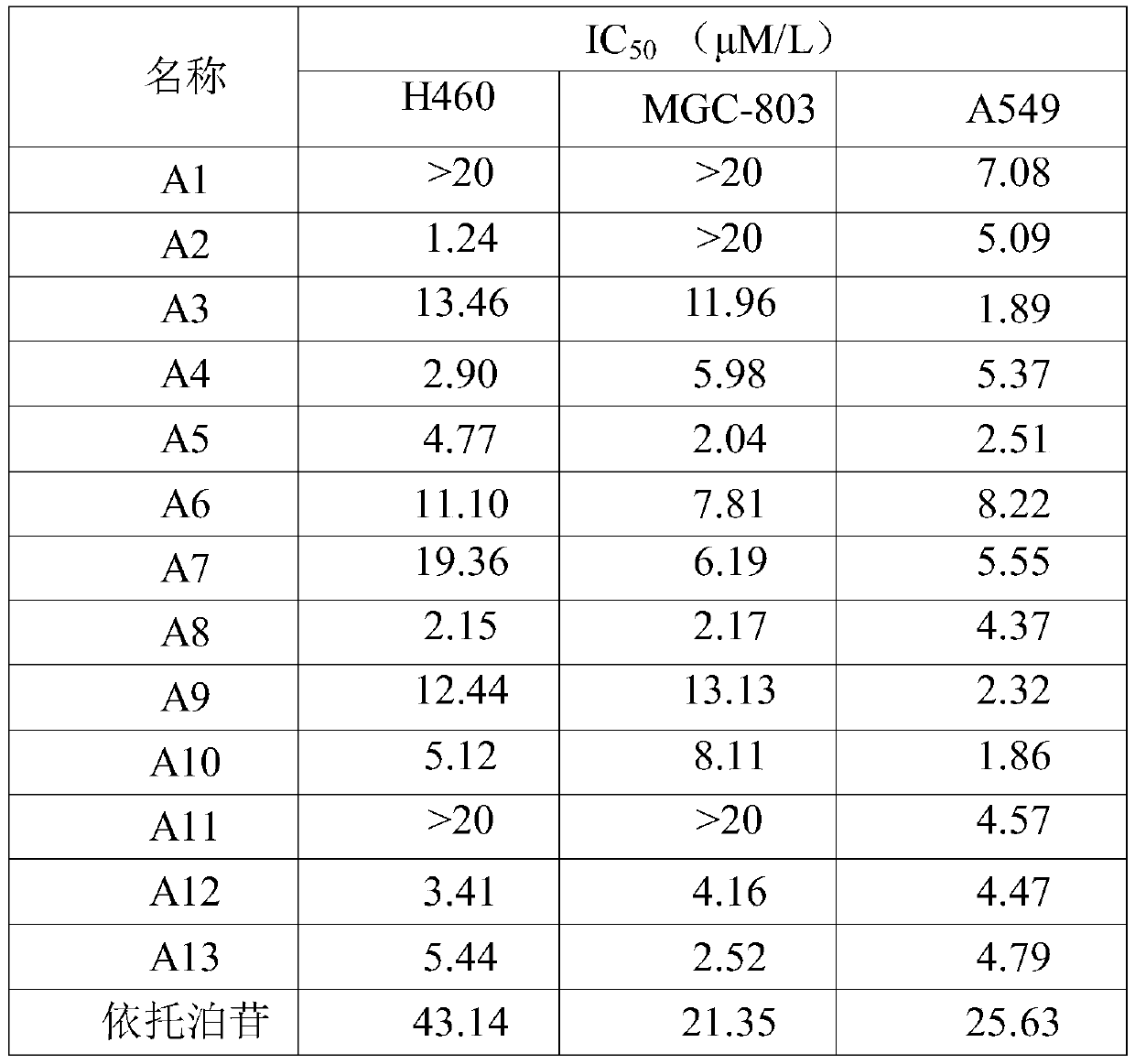
[0038] N-(3-(5-phenyl-1,3,4-oxadiazole)phenyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-sulfonamide ( A1) Preparation
[0039] a. Preparation of uracil-5-sulfonyl chloride
[0040]Add uracil (5g, 44.61mmoL) and thionyl chloride (13.27g, 111.52mmoL) into a 100mL reaction flask, react at room temperature for 1h, then add chlorosulfonic acid (17.73g, 133.82mmoL) and react at 60°C for 1h, 70 ° C to continue the reaction for 8h. After the reaction was completed, the reaction solution was added to the mixture of glacial acetic acid and ice water to precipitate a precipitate, which was filtered by suction, washed with water several times, and dried to obtain 7.25 g of white solid, yield: 77.21%.
[0041] b. Preparation of m-nitrobenzohydrazide
[0042] Add m-nitrobenzoic acid (5g, 29.92mmol), acetonitrile 50ml, potassium carbonate (10.34g, 74.80mmol), dimethyl sulfate (5.66g, 44.88mmol) in a 100ml reaction flask, react at 50°C, thin-layer chromatography Monitor the progress of th...
Embodiment 2
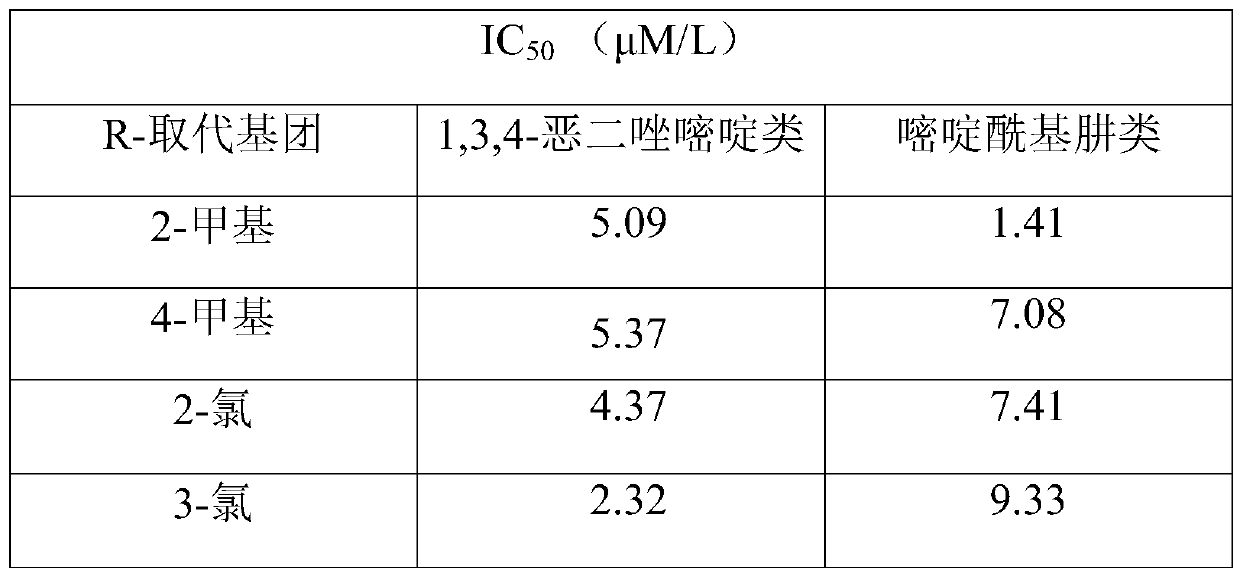
[0054] N-(3-(5-(o-tolyl)-1,3,4-oxadiazole)phenyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5- Preparation of sulfonamide (A2)
[0055] Using o-toluic acid as a raw material, 3-(5-(o-tolyl)-1,3,4-oxadiazole)aniline was prepared according to the steps of Example 1c, 1d, and 1e, and prepared according to the steps of Example 1f N-(3-(5-(o-tolyl)-1,3,4-oxadiazol-2-yl)phenyl)-2,4-dioxo-1,2,3,4-tetrahydro Pyrimidine-5-sulfonamide (A2), off-white solid, yield: 42.56%.
[0056] 1 H NMR (600MHz, DMSO-d 6 )δ11.92(s,1H),11.65(s,1H),10.58(s,1H),8.17(s,1H),8.01(d,J=7.3Hz,1H),7.88(s,1H), 7.76(d, J=7.3Hz, 1H), 7.52(d, J=4.4Hz, 2H), 7.48–7.43(m, 2H), 7.39(d, J=7.4Hz, 1H), 2.67(s, 3H ).MS(ESI,m / z):424.08[M-H] - .
Embodiment 3
[0058] N-(3-(5-(m-tolyl)-1,3,4-oxadiazole)phenyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5- Preparation of sulfonamide (A3)
[0059] Using m-toluic acid as a raw material, 3-(5-(m-tolyl)-1,3,4-oxadiazole)aniline was prepared according to the steps of Example 1c, 1d, and 1e, and prepared according to the steps of Example 1f N-(3-(5-(m-tolyl)-1,3,4-oxadiazole)phenyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5- Sulfonamide (A3), off-white solid, yield: 37.58%.
[0060] 1 H NMR (600MHz, DMSO-d 6 )δ11.91(s,1H),11.65(s,1H),10.56(s,1H),8.18(s,1H),7.89–7.85(m,3H),7.77(d,J=7.4Hz,1H ),7.53–7.48(m,2H),7.43(d,J=7.2Hz,1H),7.39(d,J=7.7Hz,1H),2.40(s,3H).MS(ESI,m / z) :424.08[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com