Pharmaceutical composition
A composition and drug technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of heart failure without obvious curative effect and unsatisfactory effect, so as to improve the control rate of blood pressure, reduce the risk of high blood pressure, and have few side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A compound amlodipine hydrochlorothiazide telmisartan folic acid capsule (1000 capsules), each containing 5 mg amlodipine / 40 mg telmisartan / 12.5 mg hydrochlorothiazide / 1 mg folic acid.
[0036] (1) Preparation of amlodipine besylate sustained-release pellets:
[0037] Amlodipine besylate sustained-release pellets include drug-containing pills and a sustained-release layer; the weight of specific raw materials is as follows:
[0038] Drug-containing pills: 6.93g of amlodipine besylate, 1.5g of povidone (PVP) K30, 50g of sucrose-starch core; sustained-release layer: Eudragit NE30D 11.75g, lactose 0.8g, talc 0.95g.
[0039] The preparation process is as follows:
[0040] (a) Weigh 6.93g of amlodipine besylate, containing 5.0g of amlodipine, and dissolve it in 100ml of ethanol; weigh 1.5g of PVP K30 and dissolve it in 50ml of water, and mix at high speed for 5min; slowly add ammonium chloride to the aqueous solution of PVP K30 Dipine benzene sulfonate solution, stirring w...
Embodiment 2
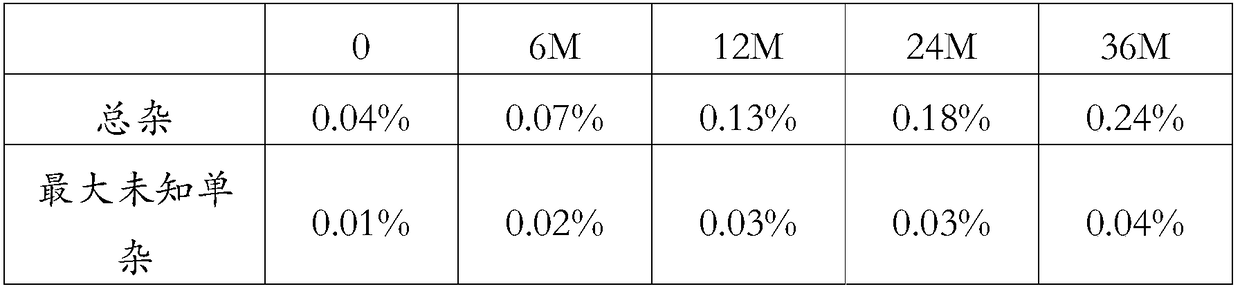
[0054] Study on Stability of Compound Amlodipine Hydrochlorothiazide Telmisartan Folic Acid Capsules
[0055] The compound amlodipine hydrochlorothiazide telmisartan folic acid capsules prepared according to Example 1 was investigated in a long-term stability test at 25° C. / 60% RH humidity, and the results were as follows:
[0056]
[0057] Experiments have shown that compound amlodipine hydrochlorothiazide telmisartan folic acid capsules have good stability.
Embodiment 3
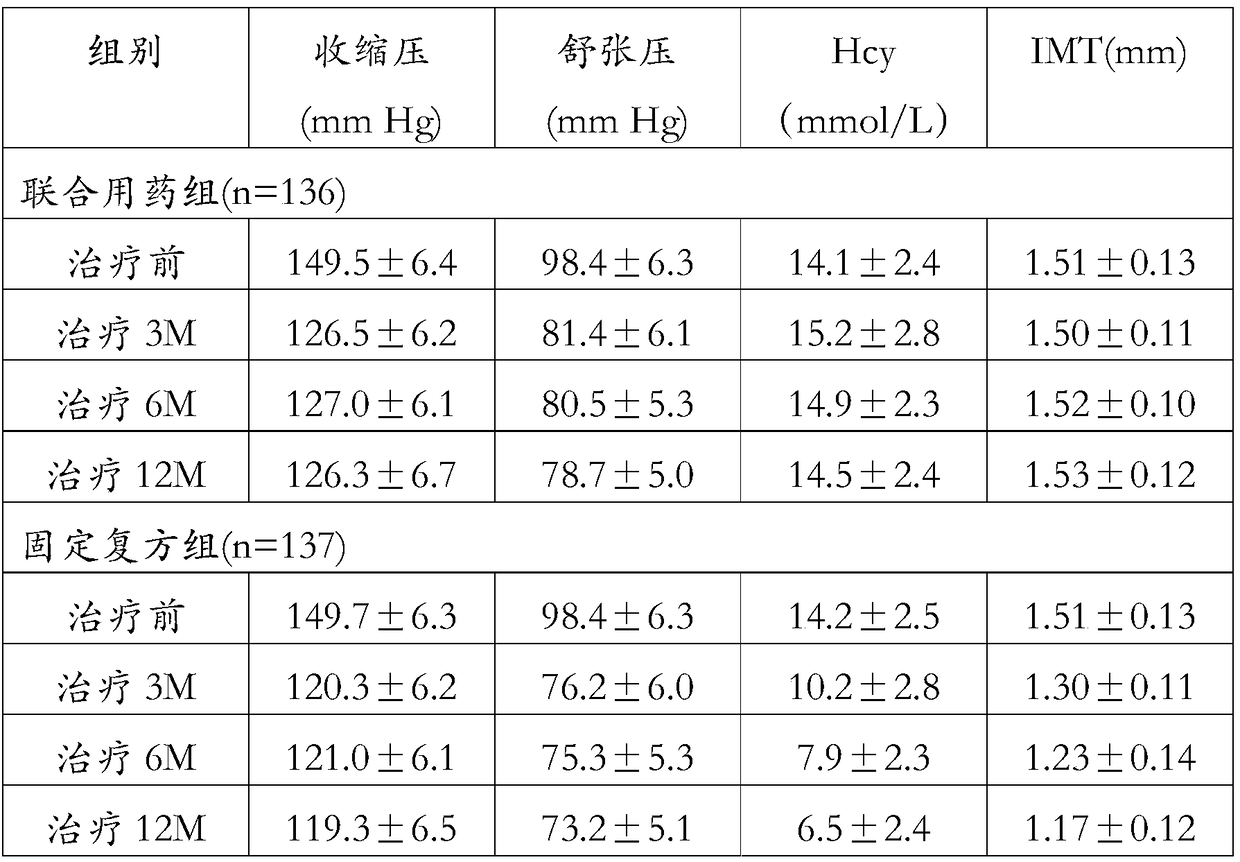
[0059] Research objects and random grouping 300 patients with H-type hypertension were selected as the research objects, and were divided into the combined drug group and the fixed compound group according to the random number table method. Finally, 273 cases were included in the research objects, including 160 males and 113 females; aged 40 -85 years old, mean (60±6.5 years old).
[0060] There were 136 cases in the combined treatment group and 137 cases in the fixed compound group. Combination drugs take unilateral amlodipine 5mg, telmisartan 40mg, hydrochlorothiazide 12.5mg, folic acid 1mg once a day respectively, and the fixed compound group takes compound amlodipine hydrochlorothiazide telmisartan folic acid prepared according to Example 1 once a day capsule. Patients with coronary heart disease are routinely treated with anti-platelet and lipid-lowering treatments.
[0061] Observation indexes and serum Hcy (homocysteine), IMT (carotid artery intima-media thickness), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com