A kind of medicine for preventing and treating coronary heart disease and preparation method thereof
A technology for drugs and cardiovascular and cerebrovascular diseases, applied in the field of medicinal chemistry, can solve problems such as ineffective treatment of coronary heart disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
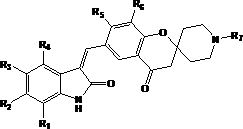
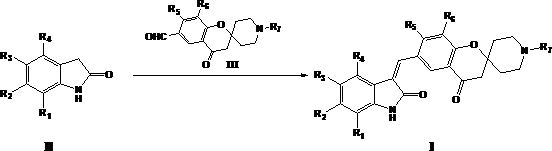
[0045] The present invention also provides a preparation method of the compound of formula I, the preparation method comprising the following steps:
[0046] Reaction of a compound of formula II with a compound of formula III in the presence of a base to prepare a compound of formula I
[0047]
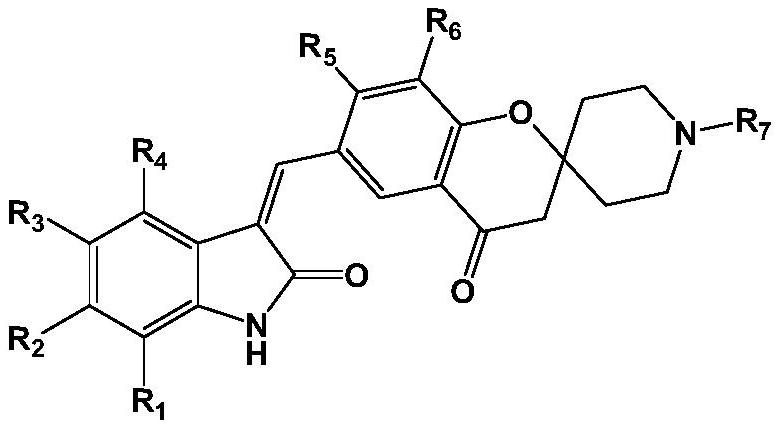
[0048] Among them, R 1 -R 7 as defined herein;
[0049] The base is selected from organic bases such as triethylamine or pyridine; or inorganic bases such as sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate.
[0050] The present invention also relates to a pharmaceutical composition comprising at least one of said compounds or pharmaceutically acceptable salts, stereoisomers, tautomers, solvates, and prodrugs thereof as an active ingredient. The pharmaceutical composition may comprise a pharmaceutically acceptable carrier or excipient.
[0051] The pharmaceutical composition can be prepared according to methods known in the art. Any dosage form su...
Embodiment 1
[0062] Example 1: (Z)-6-((6-methyl-2-oxoindole-3-methylene)methyl)-4-oxospiro[chroman-2,4'-piperidine ]-1'-tert-butyl formate (compound SP-1)
[0063]
[0064] Put tert-butyl 6-formyl-4-oxospiro[chroman-2,4'-piperidine]-1'-carboxylate (10.0mmol) into the flask, add 100m absolute ethanol to dissolve it, and then Then 6-methylindolin-2-one (10.0 mmol) and 2 ml of triethylamine were added, and the reaction was stirred at room temperature for 3 h, and a large amount of precipitation appeared. Suction filtration under reduced pressure, the filter cake was washed with a small amount of absolute ethanol, and dried under vacuum to obtain 4.34 g of the title product as a white solid, with a yield of 91.5%.
[0065] ESI-MS: 475.22[M+H] +
[0066] Elemental analysis: theoretical value C, 70.87; H, 6.37; N, 5.90; O, 16.86
[0067] Found value C, 70.58; H, 6.55; N, 5.79; O, 17.08
[0068] 1 H NMR (400MHz, CDCl 3 )δ11.08(s,1H),7.85(d,1H),7.70(s,1H),7.51(d,1H),7.39(d,1H),7.13(s,1H)...
Embodiment 2
[0069] Example 2: (Z)-6-((5,6-dimethoxy-2-oxoindoline-3-methylene)methyl)spiro[chroman-2,4'-piperidine ]-4-one (SP-2)
[0070]
[0071] According to the method of Example 1, replace 6-formyl-4-oxospiro[chroman-2,4'-piperidine]-6-carbaldehyde with 4-oxospiro[chroman-2,4'-piperidine Pyridine]-1'-carboxylic acid tert-butyl ester, substituting 5,6-dimethoxyindolin-2-one for 6-methylindolin-2-one afforded the title compound as a gray solid, yield 87.9 %.
[0072] ESI-MS: 423.18[M+H] +
[0073] Elemental analysis: theoretical value C, 68.23; H, 6.20; N, 6.63; O, 18.94
[0074] Found value C, 68.35; H, 6.02; N, 6.97; O, 18.66
[0075] 1 H NMR (400MHz, CDCl 3 )δ11.10(s,1H),7.88(d,1H),7.53(s,1H),7.29(s,1H),7.22(s,1H),7.10(d,1H),6.89(d,1H ), 3.85(s,6H), 2.85(t,4H), 2.66(s,2H), 2.02(s,1H), 1.91(t,4H).
[0076] According to a method similar to Example 1, the following compounds were synthesized:
[0077]
[0078] Example of efficacy
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com