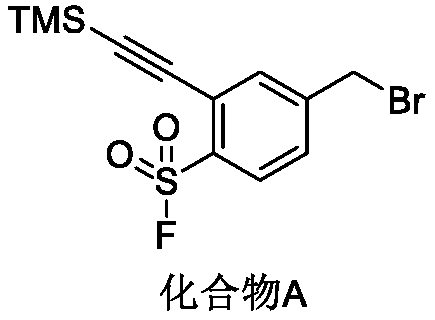
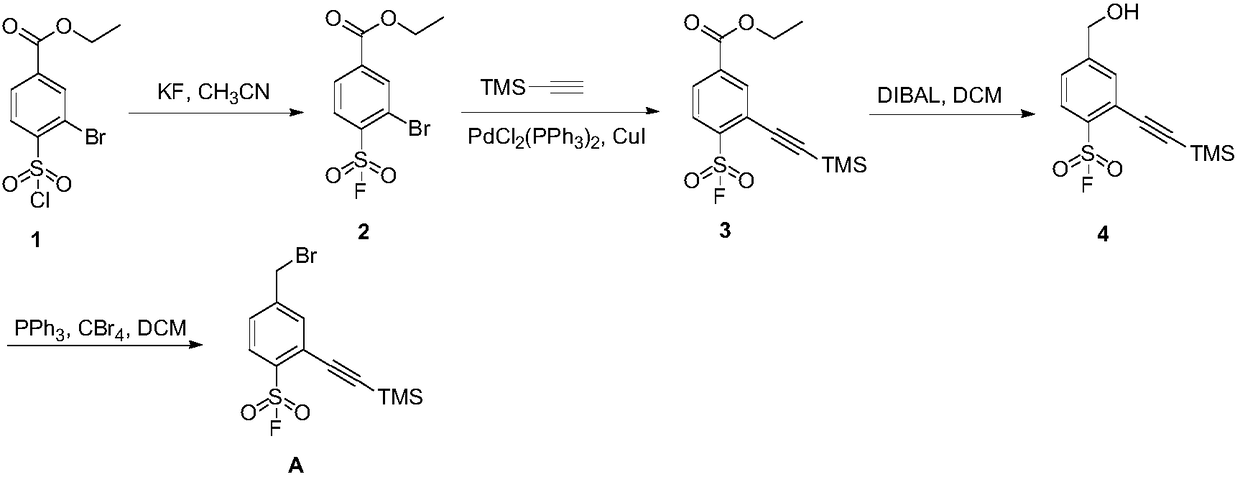
Preparation method of 4-bromoethyl-2-trimethylsilyl ethynylbenzene-1-sulfuryl fluoride
A technology of trimethylsilyl and ethynylbenzene, applied in the field of preparation of 4-bromomethyl-2-trimethylsilylethynylbenzene-1-sulfonyl fluoride, which can solve the unfavorable environmental safety and sustainable development of waste liquid , high preparation cost, many steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
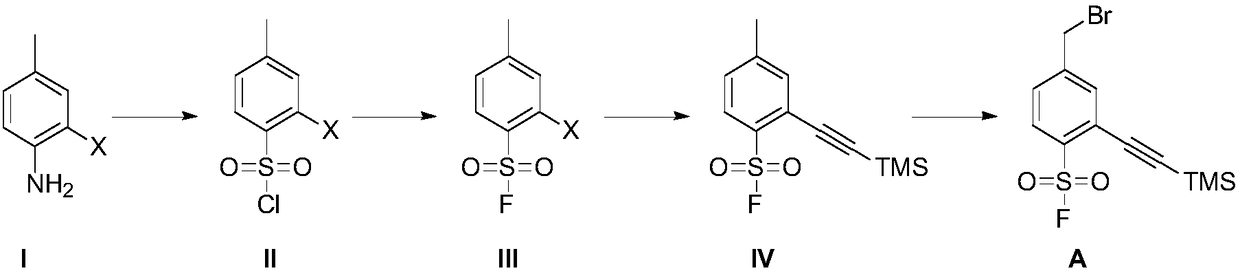
[0093] Embodiment 1, the preparation of compound II-1
[0094]
[0095] Add glacial acetic acid (300mL) and cuprous chloride (13.9g) successively in the reaction vessel, feed sulfur dioxide gas at room temperature for 1 hour, cool to 0°C to obtain reaction solution A; add compound I- 1 (13.0g), glacial acetic acid (100mL) and concentrated hydrochloric acid (12mL), stirred and cooled to 0°C, added sodium nitrite (5.3g), stirred for 1 hour to obtain reaction solution B; Added into the reaction solution A, the obtained mixed reaction solution was stirred for 2 hours, extracted with ethyl acetate, washed with water after the combined organic phase, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain compound II-1 (yellow solid, 13.3 g, yield 70%).
[0096] In other implementations, the starting material 2-bromo-4-methylaniline (compound I-1) can be replaced by 2-chloro-4-methylaniline or 2-iodo-4-methylaniline.
Embodiment 2
[0097] Embodiment 2, the preparation of compound III-1
[0098]
[0099] Compound II-1 (6.0 g), acetonitrile (40 mL) and potassium fluoride (13.0 g) were sequentially added into the reaction vessel, and the reaction solution was stirred at 25°C for 12 hours. The reaction solution was filtered, and the filtrate was concentrated. The concentrated residue was purified by column chromatography to obtain compound III-1 (light yellow solid, 4.8 g, yield 85%).
[0100] In other implementations, potassium fluoride may be replaced by sodium fluoride, cesium fluoride, or tetrabutylammonium fluoride.
[0101] Acetonitrile can be replaced by N,N-dimethylformamide and N,N-dimethylacetamide.
Embodiment 3
[0102] Embodiment 3, the preparation of compound IV
[0103]
[0104] Compound III-1 (4.5 g) and tetrahydrofuran (40 mL) were added into the reactor, followed by trimethylsilyne (6.1 g), cuprous iodide (340 mg) and triethylamine (9.0 g) in sequence. The reaction solution was stirred under nitrogen atmosphere at 25°C for 20 minutes, and then the catalyst bistriphenylphosphine palladium dichloride (2.5 g) was added, and stirred at 70°C for 12 hours. The reaction solution was concentrated, and the concentrated residue was purified by column chromatography to obtain compound IV (yellow solid, 4.0 g, yield 83%).
[0105] In other implementation cases, the above tetrahydrofuran can be replaced by toluene, ethylene glycol dimethyl ether, 1,4-dioxane, acetonitrile, ethyl acetate, N,N-dimethylformamide;
[0106] Triethylamine can be replaced by diisopropylethylamine;
[0107] Bistriphenylphosphinepalladium dichloride can be replaced by tetrakistriphenylphosphinepalladium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com