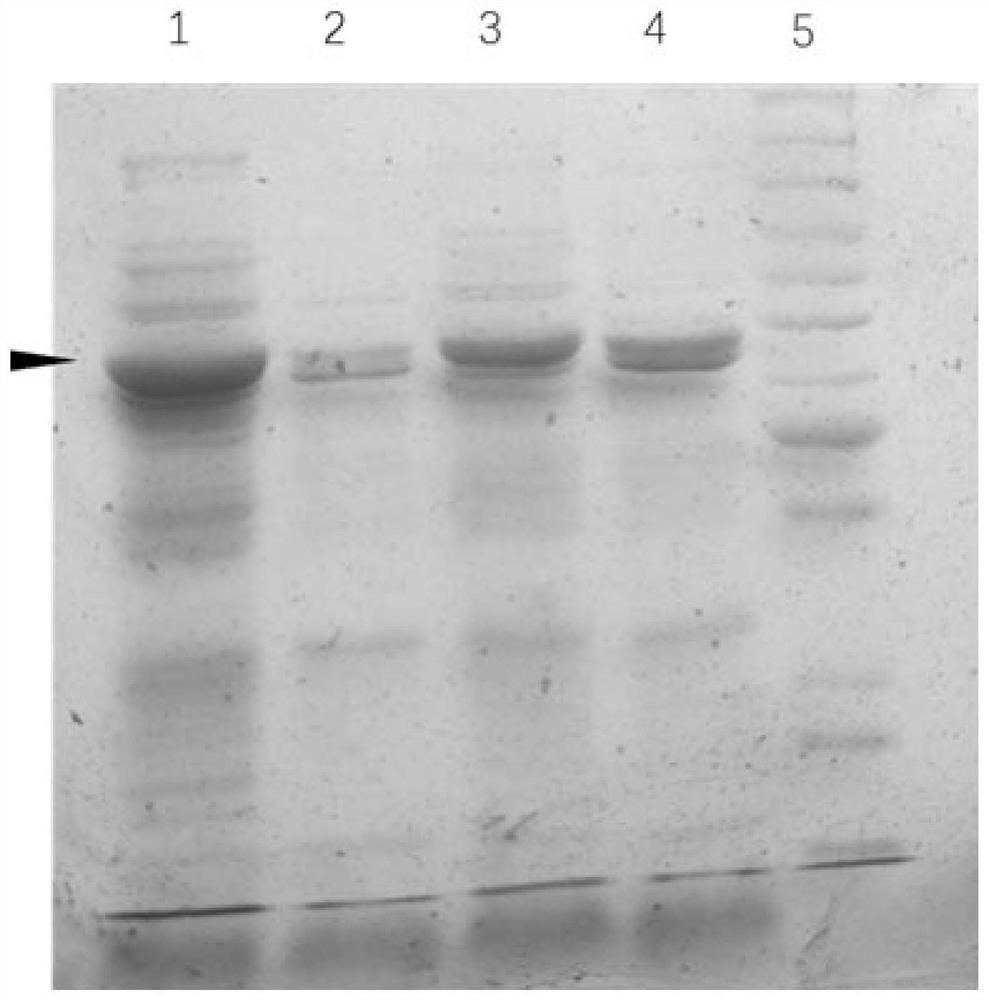
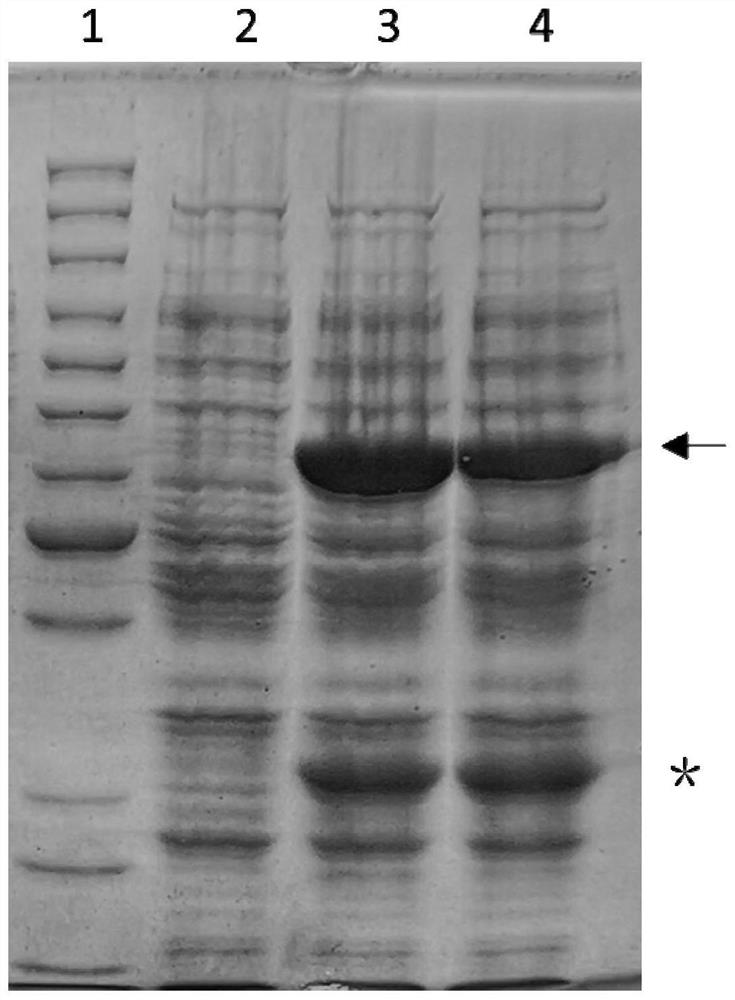
Method for one-step purification of immobilized α-amino acid fatty acyltransferase
An amino acid fatty acyl and ester acyl transferase technology, applied in transferase, biochemical equipment and methods, immobilized on or in biological cells, etc. efficiency issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Gene acquisition and vector construction
[0027] The NCBI website obtained the gene sequence of Sphingobacterium α-amino acid fatty acyltransferase (SEAT gene, Accession No.AB610978.1) and the gene sequence of Streptococcus pneumoniae formylglycine synthetase (FGE gene, Accession No.NC_000962.3), Send to a third-party service agency to synthesize the full-length gene sequence, in which the nucleotide sequence encoding LCTPSR 5'CGAATTTTTCTGTCCTCAAAGAT3' (SEQ ID NO: 2) is added before the stop codon at the 3' end of the SEAT gene, followed by BamHI and HindIII on both sides of the gene Restriction site, add CACC before the start codon of FGE gene. The synthesized SEAT gene was ligated into the pET30a vector by restriction endonuclease ligation to obtain the SEAT-30a vector.
[0028] Using the Gateway system, the FGE gene was first connected to the Topo vector, and then recombined into the PDEST17 vector to obtain the FGE-pdest17 vector. Sequencing confirmed t...
Embodiment 2
[0030] Example 2 Obtaining of expression strains
[0031] Take 100ng each of SEAT-30a and FGE-pdest17 plasmids confirmed by sequencing, mix with 100μl BL21(DE3) competent cells, let stand on ice for 30min, heat shock at 42°C for 30sec, let stand at room temperature for 5min, then add liquid LB medium Shake culture at 37°C for 30min, centrifuge at 5000rpm for 1min, discard the supernatant, resuspend the pellet with 200μl sterile water, and coat a solid LB plate containing 50μg / mL ampicillin and 30μg / mL kanamycin. Incubate overnight at 37°C. The next day, pick positive single clones, culture overnight in liquid LB medium containing 50 μg / mL ampicillin and 30 μg / mL kanamycin, add IPTG to make the final concentration 1 mM / L, and induce protein expression for 4 hours at 25 °C . Take 1mL culture solution and centrifuge at 12000g for 1min, resuspend in 100μl distilled water, add 100μl 2×SDS protein loading buffer, mix well, heat at 95°C for 10min, centrifuge at 12000g for 5min, tak...
Embodiment 3
[0032] Example 3 Protein induced fermentation
[0033] Take the SQ05 strain and culture it overnight at 37°C in liquid LB medium containing 50 μg / ml ampicillin and 30 μg / mL kanamycin. The next day, take 20ml of culture solution and further expand the culture to 1L. When the cell concentration reaches OD 600 =0.6-0.8, add IPTG to make the final concentration 0.5mM. Induced at 25°C for 6h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com